Now Reading: Efficient mitochondrial A-to-G base editors for the generation of mitochondrial disease models
-
01
Efficient mitochondrial A-to-G base editors for the generation of mitochondrial disease models
Efficient mitochondrial A-to-G base editors for the generation of mitochondrial disease models

Wallace, D. C. Mitochondrial genetic medicine. Nat. Genet. 50, 1642–1649 (2018).
Kim, J. S. & Chen, J. Base editing of organellar DNA with programmable deaminases. Nat. Rev. Mol. Cell Biol. 25, 34–45 (2024).
Cho, S. I. et al. Targeted A-to-G base editing in human mitochondrial DNA with programmable deaminases. Cell 185, 1764–177 (2022).
Li, G. et al. Gene editing and its applications in biomedicine. Sci. China Life Sci. 65, 660–700 (2022).
Silva-Pinheiro, P. & Minczuk, M. The potential of mitochondrial genome engineering. Nat. Rev. Genet. 23, 199–214 (2022).
Stewart, J. B. Current progress with mammalian models of mitochondrial DNA disease. J. Inherit. Metab. Dis. 44, 325–342 (2021).
Russell, O. M., Gorman, G. S., Lightowlers, R. N. & Turnbull, D. M. Mitochondrial diseases: hope for the future. Cell 181, 168–188 (2020).
Bayona-Bafaluy, M. P., Blits, B., Battersby, B. J., Shoubridge, E. A. & Moraes, C. T. Rapid directional shift of mitochondrial DNA heteroplasmy in animal tissues by a mitochondrially targeted restriction endonuclease. Proc. Natl Acad. Sci. USA 102, 14392–14397 (2005).
Gammage, P. A. et al. Genome editing in mitochondria corrects a pathogenic mtDNA mutation in vivo. Nat. Med. 24, 1691–1695 (2018).
Bacman, S. R. et al. MitoTALEN reduces mutant mtDNA load and restores tRNAAla levels in a mouse model of heteroplasmic mtDNA mutation. Nat. Med. 24, 1696–1700 (2018).
Zekonyte, U. et al. Mitochondrial targeted meganuclease as a platform to eliminate mutant mtDNA in vivo. Nat. Commun. 12, 3210 (2021).
Rees, H. A. & Liu, D. R. Base editing: precision chemistry on the genome and transcriptome of living cells. Nat. Rev. Genet. 19, 770–788 (2018).
Mok, B. Y. et al. A bacterial cytidine deaminase toxin enables CRISPR-free mitochondrial base editing. Nature 583, 631–637 (2020).
Huang, J. et al. Discovery of deaminase functions by structure-based protein clustering. Cell 186, 3182–3195 (2023).
Mi, L. et al. DddA homolog search and engineering expand sequence compatibility of mitochondrial base editing. Nat. Commun. 14, 874 (2023).
Guo, J. et al. A DddA ortholog-based and transactivator-assisted nuclear and mitochondrial cytosine base editors with expanded target compatibility. Mol. Cell 83, 1710–1724 (2023).
Sun, H. et al. Developing mitochondrial base editors with diverse context compatibility and high fidelity via saturated spacer library. Nat. Commun. 14, 6625 (2023).
Lim, K., Cho, S. I. & Kim, J. S. Nuclear and mitochondrial DNA editing in human cells with zinc finger deaminases. Nat. Commun. 13, 366 (2022).
Willis, J. C. W., Silva-Pinheiro, P., Widdup, L., Minczuk, M. & Liu, D. R. Compact zinc finger base editors that edit mitochondrial or nuclear DNA in vitro and in vivo. Nat. Commun. 13, 7204 (2022).
Lee, S., Lee, H., Baek, G. & Kim, J. S. Precision mitochondrial DNA editing with high-fidelity DddA-derived base editors. Nat. Biotechnol. 41, 378–386 (2023).
Phan, H. T. L., Lee, H. & Kim, K. Trends and prospects in mitochondrial genome editing. Exp. Mol. Med. 55, 871–878 (2023).
Yi, Z. et al. Strand-selective base editing of human mitochondrial DNA using mitoBEs. Nat. Biotechnol. 42, 498–509 (2024).
Hu, J. et al. Strand-preferred base editing of organellar and nuclear genomes using CyDENT. Nat. Biotechnol. 42, 936–945 (2024).
Cho, S. I. et al. Engineering TALE-linked deaminases to facilitate precision adenine base editing in mitochondrial DNA. Cell 187, 95–109 (2024).
Zhang, X. et al. Precise modelling of mitochondrial diseases using optimized mitoBEs. Nature 639, 735–745 (2025).
Fan, Y. et al. Leveraging base excision repair for efficient adenine base editing of mitochondrial DNA. Nat. Biotechnol. https://doi.org/10.1038/s41587-025-02608-w (2025).
Gorman, G. S. et al. Mitochondrial diseases. Nat. Rev. Dis. Primers 2, 16080 (2016).
Gaudelli, N. M. et al. Programmable base editing of A*T to G*C in genomic DNA without DNA cleavage. Nature 551, 464–471 (2017).
Zhou, C. et al. Off-target RNA mutation induced by DNA base editing and its elimination by mutagenesis. Nature 571, 275–278 (2019).
Grunewald, J. et al. CRISPR DNA base editors with reduced RNA off-target and self-editing activities. Nat. Biotechnol. 37, 1041–1048 (2019).
Rees, H. A., Wilson, C., Doman, J. L. & Liu, D. R. Analysis and minimization of cellular RNA editing by DNA adenine base editors. Sci. Adv. 5, eaax5717 (2019).
Richter, M. F. et al. Phage-assisted evolution of an adenine base editor with improved Cas domain compatibility and activity. Nat. Biotechnol. 38, 883–891 (2020).
Gaudelli, N. M. et al. Directed evolution of adenine base editors with increased activity and therapeutic application. Nat. Biotechnol. 38, 892–900 (2020).
Chen, L. et al. Re-engineering the adenine deaminase TadA-8e for efficient and specific CRISPR-based cytosine base editing. Nat. Biotechnol. 41, 663–672 (2023).
Chen, L. et al. Engineering a precise adenine base editor with minimal bystander editing. Nat. Chem. Biol. 19, 101–110 (2023).
Jeong, Y. K. et al. Adenine base editor engineering reduces editing of bystander cytosines. Nat. Biotechnol. 39, 1426–1433 (2021).
Tu, T. et al. A precise and efficient adenine base editor. Mol. Ther. 30, 2933–2941 (2022).
Lapinaite, A. et al. DNA capture by a CRISPR-Cas9-guided adenine base editor. Science 369, 566–571 (2020).
Arbab, M. et al. Determinants of Base Editing Outcomes from Target Library Analysis and Machine Learning. Cell 182, 463–480 e430 (2020).
Kim, H. S., Jeong, Y. K., Hur, J. K., Kim, J. S. & Bae, S. Adenine base editors catalyze cytosine conversions in human cells. Nat. Biotechnol. 37, 1145–1148 (2019).
Mok, B. Y. et al. CRISPR-free base editors with enhanced activity and expanded targeting scope in mitochondrial and nuclear DNA. Nat. Biotechnol. 40, 1378–1387 (2022).
Bacman, S. R. et al. Specific elimination of mutant mitochondrial genomes in patient-derived cells by mitoTALENs. Nat. Med. 19, 1111–1113 (2013).
Yan, D. et al. High-efficiency and multiplex adenine base editing in plants using new TadA variants. Mol. Plant 14, 722–731 (2021).
Catarino, C. B. et al. Characterization of a Leber’s hereditary optic neuropathy (LHON) family harboring two primary LHON mutations m.11778G>A and m.14484T>C of the mitochondrial DNA. Mitochondrion 36, 15–20 (2017).
Macmillan, C. et al. Pedigree analysis of French Canadian families with T14484C Leber’s hereditary optic neuropathy. Neurology 50, 417–422 (1998).
Thorburn, D. R., Rahman, J., and Rahman, S. Mitochondrial DNA-Associated Leigh Syndrome and NARP. In GeneReviews((R)), M. P. Adam, J. Feldman, G. M. Mirzaa, R. A. Pagon, S. E. Wallace, L. J. H. Bean, K. W. Gripp, and A. Amemiya, eds. (1993).
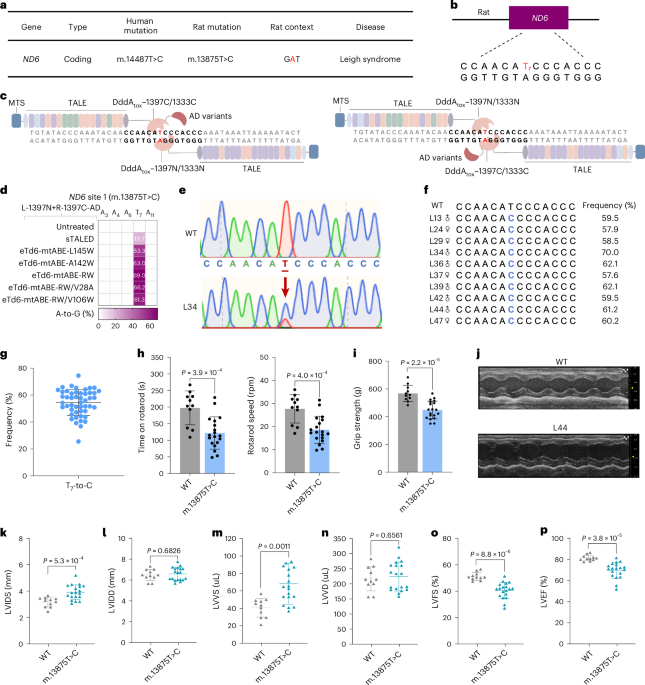
Khoo, A. et al. Progressive myoclonic epilepsy due to rare mitochondrial ND6 mutation, m.14487T>C. BMJ Neurol. Open 3, e000180 (2021).
Becker, S. & Boch, J. TALE and TALEN genome editing technologies. Gene and Genome Editing 2, 100007 (2021).
Kytovuori, L., Gardberg, M., Majamaa, K. & Martikainen, M. H. The m.7510T>C mutation: Hearing impairment and a complex neurologic phenotype. Brain Behav. 7, e00859 (2017).
Mutai, H., Watabe, T., Kosaki, K., Ogawa, K. & Matsunaga, T. Mitochondrial mutations in maternally inherited hearing loss. BMC Med. Genet 18, 32 (2017).
Ding, Y. et al. The role of mitochondrial DNA mutations in hearing loss. Biochem. Genet. 51, 7–8 (2013).
Xiao, Y. L., Wu, Y. & Tang, W. An adenine base editor variant expands context compatibility. Nat. Biotechnol. 42, 1442–1453 (2024).
Jiang, F. & Doudna, J. A. CRISPR-Cas9 Structures and Mechanisms. Annu. Rev. Biophys. 46, 505–529 (2017).
Yin, L., Shi, K. & Aihara, H. Structural basis of sequence-specific cytosine deamination by double-stranded DNA deaminase toxin DddA. Nat. Struct. Mol. Biol. 30, 1153–1159 (2023).
Kim, Y. B. et al. Increasing the genome-targeting scope and precision of base editing with engineered Cas9-cytidine deaminase fusions. Nat. Biotechnol. 35, 371–376 (2017).
Chen, L. et al. A mitochondrial disease model is generated and corrected using engineered base editors in rat zygotes. Nat. Biotechnol. https://doi.org/10.1038/s41587-025-02684-y (2025).
Chen, L. et al. Adenine transversion editors enable precise, efficient A•T-to-C•G base editing in mammalian cells and embryos. Nat. Biotechnol. 42, 638–650 (2024).
Zhang, X. et al. Increasing the efficiency and targeting range of cytidine base editors through fusion of a single-stranded DNA-binding protein domain. Nat. Cell Biol. 22, 740–750 (2020).
Chen, Y. et al. Generation of obese rat model by transcription activator-like effector nucleases targeting the leptin receptor gene. Sci. China Life Sci. 60, 152–157 (2017).
Cheng, Y. et al. Degraded cortical temporal processing in the valproic acid-induced rat model of autism. Neuropharmacology 209, 109000 (2022).
Hwang, G. H. et al. Web-based design and analysis tools for CRISPR base editing. BMC Bioinformatics 19, 542 (2018).