Now Reading: Circular RNA aptamers targeting neuroinflammation ameliorate Alzheimer disease phenotypes in mouse models
-
01
Circular RNA aptamers targeting neuroinflammation ameliorate Alzheimer disease phenotypes in mouse models
Circular RNA aptamers targeting neuroinflammation ameliorate Alzheimer disease phenotypes in mouse models
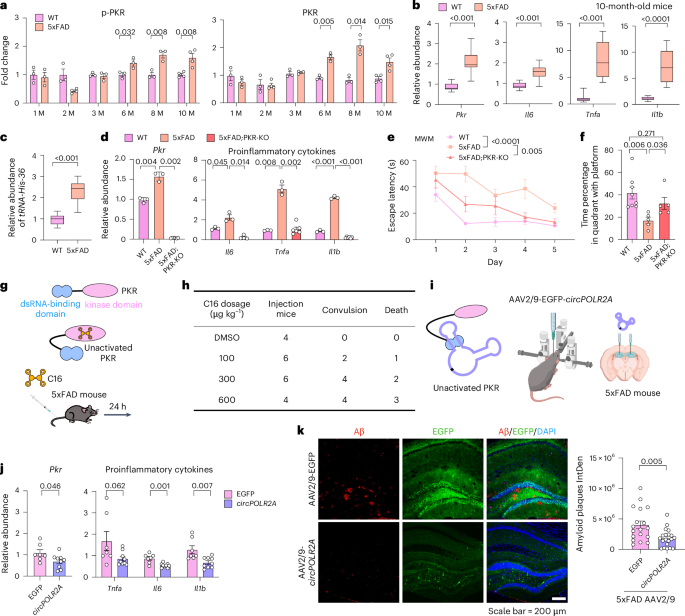
Data availability
Mouse microglia RNA-seq datasets are available from the Gene Expression Omnibus (GSE249440). The mm10 reference genome and gencode vM25 annotation GTF file were download from the GENCODE database (GRCm38.p6). Data on neuron and microglia proportions in the mouse cortex and hippocampus were obtained from the mouse whole-brain transcriptomic cell type atlas in the Allen Brain Cell Atlas database (https://portal.brain-map.org/atlases-and-data/bkp/abc-atlas). All original unprocessed data related to this paper were uploaded to Mendeley Data (https://doi.org/10.17632/nshppjsxd7.1). Source data are provided with this paper.
Code availability
This paper does not report original code.
References
-
Hardy, J. & Selkoe, D. J. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science 297, 353–356 (2002).
-
Holtzman, D. M., Morris, J. C. & Goate, A. M. Alzheimer’s disease: the challenge of the second century. Sci. Transl. Med. 3, 77sr71 (2011).
-
Gong, C. X., Liu, F. & Iqbal, K. Multifactorial hypothesis and multi-targets for Alzheimer’s disease. J. Alzheimers Dis. 64, S107–s117 (2018).
-
De Roeck, A., Van Broeckhoven, C. & Sleegers, K. The role of ABCA7 in Alzheimer’s disease: evidence from genomics, transcriptomics and methylomics. Acta Neuropathol. 138, 201–220 (2019).
-
Iqbal, K. & Grundke-Iqbal, I. Alzheimer’s disease, a multifactorial disorder seeking multitherapies. Alzheimers Dement. 6, 420–424 (2010).
-
Heppner, F. L., Ransohoff, R. M. & Becher, B. Immune attack: the role of inflammation in Alzheimer disease. Nat. Rev. Neurosci. 16, 358–372 (2015).
-
Sala Frigerio, C. et al. The major risk factors for Alzheimer’s disease: age, sex, and genes modulate the microglia response to Aβ plaques. Cell Rep. 27, 1293–1306 (2019).
-
Onyango, I. G. et al. Neuroinflammation in Alzheimer’s disease. Biomedicines 9, 524 (2021).
-
Leng, F. & Edison, P. Neuroinflammation and microglial activation in Alzheimer disease: where do we go from here? Nat. Rev. Neurol. 17, 157–172 (2021).
-
Cai, Z. et al. Role of blood–brain barrier in Alzheimer’s disease. J. Alzheimers Dis. 63, 1223–1234 (2018).
-
Rezai, A. R. et al. Noninvasive hippocampal blood–brain barrier opening in Alzheimer’s disease with focused ultrasound. Proc. Natl Acad. Sci. USA 117, 9180–9182 (2020).
-
Cummings, J. et al. Alzheimer’s disease drug development pipeline: 2024. Alzheimers Dement. (N. Y.) 10, e12465 (2024).
-
Kang, R. & Tang, D. PKR-dependent inflammatory signals. Sci. Signal. 5, pe47 (2012).
-
Hwang, K. D., Bak, M. S., Kim, S. J., Rhee, S. & Lee, Y. S. Restoring synaptic plasticity and memory in mouse models of Alzheimer’s disease by PKR inhibition. Mol. Brain 10, 57 (2017).
-
Lopez-Grancha, M. et al. A novel selective PKR inhibitor restores cognitive deficits and neurodegeneration in Alzheimer disease experimental models. J. Pharmacol. Exp. Ther. 378, 262–275 (2021).
-
Tible, M. et al. PKR knockout in the 5xFAD model of Alzheimer’s disease reveals beneficial effects on spatial memory and brain lesions. Aging Cell 18, e12887 (2019).
-
Zhu, P. J. et al. Suppression of PKR promotes network excitability and enhanced cognition by interferon-γ-mediated disinhibition. Cell 147, 1384–1396 (2011).
-
Chen, H. M., Wang, L. & D’Mello, S. R. A chemical compound commonly used to inhibit PKR, {8-(imidazol-4-ylmethylene)-6H-azolidino[5,4-g] benzothiazol-7-one}, protects neurons by inhibiting cyclin-dependent kinase. Eur. J. Neurosci. 28, 2003–2016 (2008).
-
Liu, C. X. et al. Structure and degradation of circular RNAs regulate PKR activation in innate immunity. Cell 177, 865–880 (2019).
-
Liu, C. X. et al. RNA circles with minimized immunogenicity as potent PKR inhibitors. Mol. Cell 82, 420–434 (2022).
-
Guo, S.-K. et al. Therapeutic application of circular RNA aptamers in a mouse model of psoriasis. Nat. Biotechnol. 43, 236–246 (2025).
-
Hugon, J., Mouton-Liger, F., Dumurgier, J. & Paquet, C. PKR involvement in Alzheimer’s disease. Alzheimers Res. Ther. 9, 83 (2017).
-
Peel, A. Activation of the cell stress kinase PKR in Alzheimer’s disease and human amyloid precursor protein transgenic mice. Neurobiol. Dis. 14, 52–62 (2003).
-
Fullerton, J. N. & Gilroy, D. W. Resolution of inflammation: a new therapeutic frontier. Nat. Rev. Drug Discov. 15, 551–567 (2016).
-
Oakley, H. et al. Intraneuronal β-amyloid aggregates, neurodegeneration, and neuron loss in transgenic mice with five familial Alzheimer’s disease mutations: potential factors in amyloid plaque formation. J. Neurosci. 26, 10129–10140 (2006).
-
Chang, R., Yee, K. L. & Sumbria, R. K. Tumor necrosis factor α inhibition for Alzheimer’s disease. J. Cent. Nerv. Syst. Dis. 9, 1179573517709278 (2017).
-
Belarbi, K. et al. TNF-α protein synthesis inhibitor restores neuronal function and reverses cognitive deficits induced by chronic neuroinflammation. J. Neuroinflammation 9, 23 (2012).
-
Jammi, N. V., Whitby, L. R. & Beal, P. A. Small molecule inhibitors of the RNA-dependent protein kinase. Biochem. Biophys. Res. Commun. 308, 50–57 (2003).
-
Zhang, X. O. et al. Complementary sequence-mediated exon circularization. Cell 159, 134–147 (2014).
-
Chan, K. Y. et al. Engineered AAVs for efficient noninvasive gene delivery to the central and peripheral nervous systems. Nat. Neurosci. 20, 1172–1179 (2017).
-
Hickman, S. E., Allison, E. K. & El Khoury, J. Microglial dysfunction and defective β-amyloid clearance pathways in aging Alzheimer’s disease mice. J. Neurosci. 28, 8354–8360 (2008).
-
Spangenberg, E. E. et al. Eliminating microglia in Alzheimer’s mice prevents neuronal loss without modulating amyloid-β pathology. Brain 139, 1265–1281 (2016).
-
Myers, A. & McGonigle, P. Overview of transgenic mouse models for Alzheimer’s disease. Curr. Protoc. Neurosci. 89, e81 (2019).
-
Reimer, L. et al. PKR kinase directly regulates Tau expression and Alzheimer’s disease-related Tau phosphorylation. Brain Pathol. 31, 103–119 (2021).
-
Ballard, C. et al. Alzheimer’s disease. Lancet 377, 1019–1031 (2011).
-
Reitz, C. & Mayeux, R. Alzheimer disease: epidemiology, diagnostic criteria, risk factors and biomarkers. Biochem. Pharmacol. 88, 640–651 (2014).
-
Kimura, R. & Ohno, M. Impairments in remote memory stabilization precede hippocampal synaptic and cognitive failures in 5xFAD Alzheimer mouse model. Neurobiol. Dis. 33, 229–235 (2009).
-
Vassalli, G., Bueler, H., Dudler, J., von Segesser, L. K. & Kappenberger, L. Adeno-associated virus (AAV) vectors achieve prolonged transgene expression in mouse myocardium and arteries in vivo: a comparative study with adenovirus vectors. Int. J. Cardiol. 90, 229–238 (2003).
-
Zabaleta, N. & Gil-Farina, I. Tracing the fate of AAV vectors in the body. Nat. Biotechnol. 42, 1183–1184 (2024).
-
Hollidge, B. S. et al. Kinetics and durability of transgene expression after intrastriatal injection of AAV9 vectors. Front. Neurol. 13, 1051559 (2022).
-
Xu, M. et al. A systematic integrated analysis of brain expression profiles reveals YAP1 and other prioritized hub genes as important upstream regulators in Alzheimer’s disease. Alzheimers Dement. 14, 215–229 (2018).
-
Lin, R. et al. Directed evolution of adeno-associated virus for efficient gene delivery to microglia. Nat. Methods 19, 976–985 (2022).
-
d’Errico, P. et al. Microglia contribute to the propagation of Aβ into unaffected brain tissue. Nat. Neurosci. 25, 20–25 (2022).
-
Chen, C.-H. et al. Increased NF-κB signalling up-regulates BACE1 expression and its therapeutic potential in Alzheimer’s disease. Int. J. Neuropsychopharmacolog. 15, 77–90 (2012).
-
Paquet, C. et al. The PKR activator PACT is induced by Aβ: involvement in Alzheimer’s disease. Brain Pathol. 22, 219–229 (2012).
-
Morel, M., Couturier, J., Lafay-Chebassier, C., Paccalin, M. & Page, G. PKR, the double stranded RNA-dependent protein kinase as a critical target in Alzheimer’s disease. J. Cell. Mol. Med. 13, 1476–1488 (2009).
-
Zunt, J. R. Central nervous system infection during immunosuppression. Neurol. Clin. 20, 1–22 (2002).
-
Bradshaw, M. J., Cho, T. A. & Chow, F. C. Central nervous system infections associated with immunosuppressive therapy for rheumatic disease. Rheum. Dis. Clin. North Am. 43, 607–619 (2017).
-
Vorhees, C. V. & Williams, M. T. Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat. Protoc. 1, 848–858 (2006).
-
Bolger, A. M., Lohse, M. & Usadel, B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30, 2114–2120 (2014).
-
Langmead, B. & Salzberg, S. L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 9, 357–359 (2012).
-
Kim, D., Paggi, J. M., Park, C., Bennett, C. & Salzberg, S. L. Graph-based genome alignment and genotyping with HISAT2 and HISAT-genotype. Nat. Biotechnol. 37, 907–915 (2019).
-
Liao, Y., Smyth, G. K. & Shi, W. featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 30, 923–930 (2014).
-
Yu, G., Wang, L. G., Han, Y. & He, Q. Y. clusterProfiler: an R package for comparing biological themes among gene clusters. OMICS 16, 284–287 (2012).
Acknowledgements
We thank B. Lu for discussion and reading the manuscript, M. Luo and R. Lin for providing packaging plasmids of AAV-MG1.2, N. Jing for gifting 5xFAD mice and S. Cai and Y. Chen for gifting PS19 mice. This work was supported by the National Key R&D Program of China (2021YFA1300501), Strategic Priority Research Program of the CAS (XDB0570000) and Science and Technology Commission of Shanghai Municipality (23DX1900100 and 23DX1900101) to L.-L.C. This work was supported by the New Cornerstone Science Foundation through the New Cornerstone Investigator Program. L.-L. Chen is a Shanghai Academy of Natural Sciences Senior Investigator.
Ethics declarations
Competing interests
L.-L.C., X.F. and C.-X.L. are named as inventors on patents related to circRNA aptamers held by the CEMCS, CAS. L.-L.C. is a scientific cofounder of RiboX Therapeutics. The other authors declare no competing interests.
Peer review
Peer review information
Nature Biotechnology thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Elevated PKR with AD progression exacerbates neuroinflammation and Aβ aggregation.
a, PKR and p-PKR levels were comparable in the hippocampus of 1-, 2-, and 3-month-old WT and 5xFAD mice but increased in 6-, 8-, and 10-month-old 5xFAD mice, compared to WT, as measured by WB (n=4 for 2- and 10-month-old 5xFAD; n=3 for others). b, Expression of Pkr, Il6, Tnfα, and Il-1β was similar in 1-, 2-, and 3-month-old mice but up-regulated in 5xFAD hippocampus at 6-, 8-, and 10-months. n = 3. Measured by RT-qPCR. c, PKR and p-PKR levels increased with age and AD progression in both WT and 5xFAD mice, detected by WB in 1- and 10-month-old mice. d, RNase L was mildly activated in the hippocampus of 5xFAD mice during AD progression. RT-qPCR was used to measure activation levels in WT and 5xFAD mice at different ages. n=6 for 6- and 10-month-old WT mice; n=9 for 6-month-old 5xFAD mice; n=7 for 10-month-old 5xFAD mice and n=3 for the others. e, PKR knockout was confirmed by WB in 5xFAD; PKR-KO mice, n=3. f, Increased phosphorylation of PKR was triggered by EMCV infection, as measured by WB, n = 4. g, Schematic showing 3-month-old 5xFAD mice received bilateral intrahippocampal injections of EMCV (103 virus genome) or PBS. Detection was conducted 48h post-injection. h, PKR activation promoted Aβ plaque accumulation. Left: Representative images of 5xFAD and 5xFAD; PKR-KO mice injected with EMCV or PBS. EMCV-infected 5xFAD mice exhibited increased Aβ plaques, while 5xFAD; PKR-KO mice showed no significant changes. DsRNA from EMCV (J2, red), Aβ plaques (green), DAPI (blue). Right: Statistical analysis (n = 3). Scale bar = 200 μm. Data analyzed by two-tailed Student’s t test. i–j, Swim speed in MWM showed no significant difference. n = 8 (WT), n = 5 (5xFAD), n = 5 (5xFAD; PKR-KO). Data analyzed by two-way ANOVA in i. k, Motor function test on rotarod showed intact motor balance in all mice. n = 8 (WT), n = 5 (5xFAD), n = 5 (5xFAD; PKR-KO). b, d, h, j and k: Two-tailed Student’s t test, error bars represent mean ± SEM.
Extended Data Fig. 2 Ds-cRNAs outcompete C16 to alleviate molecular symptoms in 5xFAD mice.
a, 300 μg/kg of C16 prevented Pkr, Il6, Tnfα, and Il-1β expression in the hippocampus of 5xFAD mice, as detected by RT-qPCR. n = 4 (DMSO), n = 5 (C16-100 μg/kg), n = 4 (C16-300 μg/kg). b, No detectable changes in glial cells were observed in the hippocampus of 5xFAD mice 24 h after injection. Astrocytes (GFAP, red), microglia (Iba1, green), activated glia (CD68, cyan) and nuclei (DAPI, blue). Scale bar = 200 μm. The right panel showed statistical analysis. n = 10 (DMSO), n = 12 (C16-300 μg/kg). c, No improvement in neuronal loss or Aβ plaque accumulation was detected in the hippocampus of 5xFAD mice 24 h after C16 injection. Magenta: NeuN, green: Aβ plaques, blue: DAPI. Scale bar = 200 μm. Right: Statistical analysis (n = 12). d, IF staining showed that AAV-EGFP mainly infected neurons (NeuN, red) and parts of microglia (Iba1, blue). Scale bar = 20 μm. Right: Colocalization analysis using Manders’ coefficients. n = 10. e, Schematic of AAV vectors used. Top: circPOLR2A vector includes POLR2A exons, 1/2 EGFP fragments, reverse complementary sequences for back splicing, and AAV inverted terminal repeats (ITR). Bottom: Control EGFP vector expressing only egfp mRNA. f, AAV infection did not activate PKR or stimulate microglia proliferation. Left: WB detected PKR phosphorylation (p-PKR), PKR, and Iba1 expression. Right: Quantification normalized to Actin. n = 4. g, AAV infection did not induce inflammatory responses in the hippocampus of WT mice, as confirmed by RT-qPCR. n = 4. h, CircPOLR2A expression in the mice with intrahippocampal injection was confirmed by Northern Blotting (NB) on denatured PAGE gel. DIG-labeled anti-sense full-length circRNA probes identified precursor RNA, circRNA, and linear RNA (weaker bands at the bottom). i, The copy number of circPOLR2A in the hippocampus of intrahippocampal injection mice was approximately 4.78×10⁷ per μg total RNA, as revealed by RT-qPCR. j, AAV2/9-circPOLR2A delivery attenuated excessive PKR phosphorylation and reduced Aβ plaques in the hippocampus of 5xFAD mice compared to EGFP controls. n = 5. a, b, c, d, f and j: Two-tailed Student’s t test, error bars represent mean ± SEM.
Extended Data Fig. 3 Delivery of ds-cRNAs into the hippocampus by intravenous injection in 5xFAD mice.
a, Intravenous injection of AAV-PHP.eB-EGFP resulted in widespread expression of EGFP across the brain, particularly in the cortex and hippocampus. EGFP (green) and DAPI-stained nuclei (blue) were visualized. Scale bar = 2.5 mm. b, NB confirmed circRNA expression following AAV-PHP.eB delivery. DIG-labeled anti-sense full-length circRNA probes detected precursor RNA, circRNA, and linear RNA (weaker bands indicated by blue arrows). c, RT-qPCR quantified the copy number of AAV-PHP.eB-delivered circRNAs per ug total RNA. d, Ds-cRNA delivery reduced PKR and pro-inflammatory cytokine expression in 5xFAD mice hippocampus compared to EGFP control. CircSMARCA5 showed no significant difference. Data presented as mean ± SEM (n = 5 for circSMARCA5 and n = 6 for others), analyzed by two-tailed Student’s t-test. e, ELISA revealed lower Aβ1-42 levels (~14,000 pg/mL) in ds-cRNA-treated groups compared to those (~26,000 pg/mL) in EGFP and non-ds-cRNA controls. Data presented as mean ± SEM (n = 4 EGFP, n = 3 circPOLR2A and circCAMSAP1, n = 5 circSMARCA5), analyzed by two-tailed Student’s t-test. f, Western blot analysis demonstrated that ds-cRNA treatment reduced the levels of PKR, p-PKR, APP, Tau, p-Tau, and pro-inflammatory cytokines in the hippocampus of 5xFAD mice, whereas EGFP treatment had no impact. g, AAV-mediated ds-cRNA delivery reduced Tau phosphorylation around Aβ plaques in 5xFAD mice. Left: Representative images of brain sections stained with Aβ plaques (Thio-S, blue) and p-Tau (AT8, red). Scale bar = 10 μm. Right: Statistical analysis from brain slices (n = 17 (5xFAD EGFP), n = 16 (5xFAD circPOLR2A), n = 20 (5xFAD circCAMSAP1), n = 27 (5xFAD circSMARCA5)), data presented as mean ± SEM, analyzed by two-tailed Student’s t-test. h, Ds-cRNA delivery suppressed astrocyte proliferation in 5xFAD mice hippocampus compared to EGFP controls. Non-ds-cRNA treatment caused no detectable change in gliosis. Left: Representative images of hippocampal sections stained with GFAP-labeled astrocytes (red) and DAPI-stained nuclei (blue). Scale bar = 100 μm. Right: Statistical analysis of GFAP-positive areas, data presented as mean ± SEM, analyzed by two-tailed Student’s t-test. (n = 30 (WT EGFP), n = 18 (5xFAD EGFP), n = 20 (5xFAD circPOLR2A), n = 20 (5xFAD circCAMSAP1), n = 28 (5xFAD circSMARCA5)).
Extended Data Fig. 4 Ds-cRNAs addition by AAV rescue AD phenotypes in PS19 mice.
a, RNase L was mildly activated in the hippocampus of 10-month-old PS19 mice (n = 5) compared to WT mice (n = 6). Data were analyzed by two-tailed Student’s t test. Box plots show the median (center line), the first and third quartiles (box bounds), and data range (whiskers). b, Schematic illustrating the experimental design. Six-month-old PS19 mice received intravenous injections of ds-cRNA, non-ds-cRNA, or EGFP, with behavioral and histological analyses performed 3 months later. c, Copy number of delivered circRNAs per µg of total RNA, as determined by RT-qPCR. d, Delivery of ds-cRNAs by AAV-PHP.eB dampened inflammatory responses in the hippocampus of PS19 mice. n = 5 (WT EGFP and PS19 circSMARCA5), n = 6 (PS19 EGFP, PS19 circPOLR2A and PS19 circCAMSAP1). e, Ds-cRNA delivery alleviated p-Tau aggregation in PS19 mice. Left: Representative images of p-Tau (AT8, red) and nuclei (DAPI, blue) staining in hippocampal sections. Scale bar = 200 μm. Right: Quantification of AT8-stained areas. n = 13 (WT EGFP), n = 14 (PS19 EGFP), n = 12 (PS19 circPOLR2A, PS19 circCMASAP1), n = 15 (PS19 circSMARCA5). f, Ds-cRNA delivery reduced microglial proliferation and neuronal loss in PS19 mice. Left: Representative images showing microglia (Iba1, red), neurons (NeuN, magenta), and nuclei (DAPI, blue). Scale bar = 200 μm. Right: Statistical analysis of microglial proliferation and neuronal loss. For Iba1, n = 50 (WT EGFP), n = 32 (PS19 EGFP), n = 26 (PS19 circPOLR2A), n = 32 (PS19 circCAMSAP1) and n = 30 (PS19 circSMARCA5). For NeuN, n = 48 (WT EGFP), n = 16 (PS19 EGFP), n = 26 (PS19 circPOLR2A), n = 31 (PS19 circCAMSAP1) and n = 31 (PS19 circSMARCA5). g, Ds-cRNA delivery reduced astrocyte proliferation in PS19 mice. Left: Representative images showing astrocytes (GFAP, red) and nuclei (DAPI, blue). Scale bar = 200 μm. Right panel: Statistical analysis of astrocyte proliferation. n = 51 (WT EGFP), n = 40 (PS19 EGFP), n = 31 (PS19 circPOLR2A), n = 8 (PS19 circCAMSAP1) and n = 18 (PS19 circSMARCA5). d, e, f, and g, data were presented as mean ± SEM and analyzed by two-tailed Student’s t test.
Extended Data Fig. 5 Ds-cRNAs addition by AAV rescue memory deficits with no difference in anxiety and motor ability in two mouse models.
a, PS19 mice injected with AAV PHP.eB-circPOLR2A or AAV PHP.eB-circCAMSAP1 exhibited improved spatial learning abilities compared to those injected with control AAV, but no improvement was observed in the non-ds-cRNA delivery group. Mice were injected intravenously with AAV-PHP.eB, and behavioral testing was conducted three months later using the hidden platform training sessions of the Morris Water Maze (MWM). Sample sizes were as following: n = 14 (WT PHP.eB-EGFP), n = 9 (PS19 PHP.eB-EGFP), n = 10 (PS19 PHP.eB-circPOLR2A), n = 9 (PS19 PHP.eB-circCAMSAP1), and n = 8 (PS19 PHP.eB-circSMARCA5). Data were analyzed by two-way ANOVA. Error bars represent mean ± SEM. b, Probe trials were conducted to assess the retention of spatial memory. Memory retention deficits were rescued by overexpression of ds-cRNA in PS19 mice. c-d, Swim speed in the Morris water maze had no significant difference. Data in (c) were analyzed by two-way ANOVA. Error bars represent mean ± SEM. e, Motor function was tested in rotarod. f-h, Open field test. Time percentage spent in center (f), moving speed (g) and total distance of movement (h) of an open field for 10 min. Behavioral mice showed no anxiety. i-k, Open field test. Time percentage spent in center (i), moving speed (j) and total distance of movement (k) of an open field for 10 min. n = 11 (WT PHP.eB-EGFP), n = 7 (5xFAD PHP.eB-EGFP), n = 13 (5xFAD PHP.eB-circPOLR2A), n = 9 (5xFAD PHP.eB-circCAMSAP1), n = 11 (5xFAD PHP.eB-circSMARCA5). Behavioral mice showed no anxiety. l-m, Swim speed in the Morris water maze had no significant difference. n, Motor function was tested in rotarod. b, d–k, m and n, data were analyzed by two-tailed Student’s t test, error bars represent mean ± SEM.
Extended Data Fig. 6 Ds-cRNAs alleviate AD-related symptoms with treatment at both early and late stages of disease.
a, Delivery of circPOLR2A reduced neuronal loss in 3-month-old 5xFAD mice during early-stage AD. Left: Representative images showing NeuN-labeled neurons (magenta) and DAPI-stained nuclei (blue). Scale bar = 200 μm. Right: Statistical analysis, n = 16 (EGFP), n = 15 (circPOLR2A). b, CircPOLR2A delivery alleviated gliosis in 3-month-old 5xFAD mice. Left: Representative images showing microglia labeled with Iba1 (red), astrocytes labeled with GFAP (magenta), and nuclei stained with DAPI (blue). Scale bar = 200 μm. Right: Statistical analysis, n = 16 slices from 3 replicates. c, In 10-month-old 5xFAD mice, delivery of circPOLR2A reduced neuronal loss compared to EGFP. Left: Representative images showing NeuN-labeled neurons (magenta) and nuclei (DAPI, blue). Scale bar = 200 μm. Right: Statistical analysis of neuronal loss (n = 10 slices from 3 mice). d, Delivery of circPOLR2A reduced gliosis in 10-month-old 5xFAD mice in the late stage of AD. Left: Representative images showing microglia labeled with Iba1 (red), astrocytes labeled with GFAP (magenta), and nuclei stained with DAPI (blue). Scale bar = 200 μm. Right: Statistical analysis of gliosis, based on 12 slices from 3 replicates. e, Schematic diagram of the experimental procedure. f, Ds-cRNA group displayed reduced phosphorylated Tau compared to the EGFP control. Left: Immunofluorescence images of hippocampal sections from 11-month-old PS19 mice injected with PHP.eB-EGFP (n = 16) or PHP.eB-circPOLR2A (n = 14). Scale bar = 200 μm. Right: The relative percentage of the area covered by AT8 staining compared with the WT group. g, AAV-mediated delivery of ds-cRNAs alleviated gliosis in PS19 mice. Left: Immunofluorescence images of hippocampal sections from 11-month-old PS19 mice injected with PHP.eB-EGFP (n = 16) or PHP.eB-circPOLR2A (n = 14), stained with GFAP (cyan), Iba1 (red), and DAPI (blue). Scale bar = 200 μm. Right: Statistical analysis. h, AAV-mediated ds-cRNA delivery led to reduced neuron loss in PS19 mice. Left: Immunofluorescence images of hippocampal sections from 11-month-old PS19 mice, stained with NeuN (magenta) and DAPI (blue). Scale bar = 200 μm. Right: Statistical analysis (n = 16 EGFP, n = 12 circPOLR2A). a-d, f-h, Mean values ± SEM were shown. Data were analyzed by two-tailed Student’s t test.
Extended Data Fig. 7 Rescued phenotypes in AD mice three months post the AAV injection.
a, Schematic diagram of the experimental procedure. b, CircPOLR2A(9,10) was expressed in the hippocampus by intravenous injection (i.v.) of AAV-PHP.eB, as confirmed by NB on denatured PAGE. c, The copy number of circPOLR2A(9,10) in the hippocampus, delivered via AAV-PHP.eB i.v., were approximately 3.91 × 107 per μg RNA, as determined by RT-qPCR. d, Ds-cRNA group displayed reduced Aβ plaques and phosphorylated Tau compared to the EGFP control group. Right: Representative immunofluorescence images of hippocampal sections from 9-month-old 5xFAD mice injected with PHP.eB-EGFP (n = 22) or PHP.eB-circPOLR2A(9,10) (n = 16). Scale bar = 200 μm. Left: The relative percentage of the area of sections taken from the hippocampus with Thio-S and AT8 staining compared with that from the WT group. e, AAV-mediated delivery of ds-cRNAs alleviated gliosis in 5xFAD mice. Left, representative immunofluorescence images of hippocampal sections from 9-month-old 5xFAD mice injected with PHP.eB-EGFP (n = 20) or PHP.eB-circPOLR2A(9,10) (n = 16). Iba1(red), GFAP (red) and DAPI (blue) were stained. Scale bar = 200 μm. Right, statistical analysis of Iba1-positive and GFAP-positive areas. f, AAV-mediated delivery of ds-cRNAs alleviated neuron loss in 5xFAD mice. Left, representative immunofluorescence images of hippocampal sections from 9-month-old 5xFAD mice injected with PHP.eB-EGFP or PHP.eB-circPOLR2A(9,10). NeuN(red) and DAPI (blue) were stained. Scale bar = 200 μm. Right, the relative percentage of the area of sections taken from the hippocampus covered by NeuN staining compared with that from the WT group. n = 25 (5xFAD PHP.eB-EGFP), n = 21 (5xFAD PHP.eB-circPOLR2A). g-h, Swim speed in MWM showed no significant difference. Data in (g) were analyzed by two-way ANOVA. i-k, Open field test. Time percentage spent in center (i), moving speed (j), and total distance of movement (k) in an open field for 10 min. n = 8 (WT PHP.eB-EGFP), n = 7 (5xFAD PHP.eB-EGFP), n = 8 (5xFAD PHP.eB-circPOLR2A), n = 7 (5xFAD PHP.eB-circSMARCA5). Behavioral mice showed no anxiety. l, Motor function test on rotarod showed intact motor balance activity of all mice. d-f and h-l, mean values ± SEMs were shown. Data were analyzed by two-tailed Student’s t test.
Extended Data Fig. 8 AAV delivery of ds-cRNAs alleviates neuroinflammation in AD mice for at least 6 months.
a, Schematic diagram of the experimental procedure. b-c, CircPOLR2A(9,10) and circCAMSAP1(2,3) were delivered to the hippocampus by intravenous injection (i.v.) of AAV-PHP.eB, as confirmed by NB on denatured PAGE. DIG-labeled anti-sense full-length circRNA was used as the probe, labeling precursor RNA, circRNA, and linear RNA (with weaker bands at the bottom, indicated by blue arrows). d, Ds-cRNA group displayed reduced Aβ plaque loads stained with Thio-S. Left, representative immunofluorescence images of hippocampal sections from 12-month-old WT mice injected with PHP.eB-EGFP (n = 19), and 5xFAD mice injected with PHP.eB-EGFP (n = 19), PHP.eB-circPOLR2A(9,10) (n = 22) or circSMARCA5(15,16) (n = 26). Scale bar = 200 μm. Right, the relative percentage of the hippocampal area stained with Thio-S compared to the WT group. e, AAV-mediated delivery of ds-cRNAs alleviated Aβ plaque accumulation and gliosis in 5xFAD mice. Left, representative immunofluorescence images of hippocampal sections from 12-month-old WT mice injected with PHP.eB-EGFP (n = 25), and 5xFAD mice injected with PHP.eB-EGFP (n = 28), PHP.eB-circPOLR2A(9,10) (n = 27), or PHP.eB-circSMARCA5(15,16) (n = 22). Aβ (cyan), Iba1 (red) and DAPI (blue) were stained. Scale bar = 200 μm. Right, statistical analysis of Iba1-positive areas. f, AAV-mediated delivery of ds-cRNAs alleviated gliosis in 5xFAD mice. Left, immunofluorescence images of hippocampal sections from 12-month-old WT mice injected with PHP.eB-EGFP (n = 24), and 5xFAD mice injected with PHP.eB-EGFP (n = 35), PHP.eB-circPOLR2A(9,10) (n = 24), or PHP.eB-circSMARCA5(15,16) (n = 26). GFAP (red) and DAPI (blue) were stained. Scale bar = 100 μm. Right, the relative percentage of the GFAP-positive area of sections taken from the hippocampus. g, AAV-mediated delivery of ds-cRNAs showed no effect on alleviating neuron loss in 5xFAD mice. Left: Representative images of hippocampal sections stained with NeuN (red) and DAPI (blue). Scale bar = 100 μm. Right: The relative percentage of the hippocampal area covered by NeuN staining compared to the WT group. n = 22 (WT EGFP), n = 18 (5xFAD EGFP), n = 21 (5xFAD circPOLR2A), n = 22 (5xFAD circSMARCA5). d-g, Mean values ± SEMs were shown. Data were analyzed by two-tailed Student’s t test.
Extended Data Fig. 9 AAV delivery of ds-cRNAs to the AD mice at early stage alleviates neuroinflammation for at least 6 months.
a, Schematic of the experimental procedure where AAV-circPOLR2A(9,10) was administered to 3-month-old 5xFAD mice. b, CircPOLR2A(9,10) delivery to the hippocampus via i.v. by AAV-PHP.eB was confirmed by NB on denatured PAGE. DIG-labeled anti-sense full-length circRNA probe revealed precursor RNA, circRNA, and linear RNA (weaker bands, blue arrows). c, Administration of AAV-circPOLR2A(9,10) decreased Aβ plaques six months after injection. Left: Aβ plaques in the hippocampus were stained with anti-Aβ (red) and DAPI (blue). Scale bar = 200 μm. Right: Quantification of Aβ plaques, n = 22 (5xFAD EGFP), n = 21 (5xFAD circPOLR2A). d-e, CircPOLR2A(9,10) delivery alleviated gliosis in 5xFAD mice 6 months after injection. Left, representative images of hippocampal sections stained with Iba1 (red), GFAP (red) and DAPI (blue). Scale bar = 100 μm. Right, quantification of Iba1 and GFAP staining areas. For Iba1: n = 21 (WT EGFP), n = 22 (5xFAD EGFP), n = 20 (5xFAD circPOLR2A). For GFAP: n = 19 (WT EGFP), n = 23 (5xFAD EGFP), n = 26 (5xFAD circPOLR2A). f, CircPOLR2A(9,10) delivery reduced neuron loss in 5xFAD mice 6 months after injection. Left: representative hippocampal images stained with NeuN (red) and DAPI (blue). Scale bar = 200 μm. Right: Quantification of NeuN staining areas, n = 16 (WT EGFP), n = 18 (5xFAD EGFP), n = 16 (5xFAD circPOLR2A). g, 5xFAD mice injected with AAV.PHP.eB-circPOLR2A(9,10) displayed no improvement in spatial learning compared to control mice. Behavioral test was performed 6 months later using MWM. n = 11 (WT PHP.eB-EGFP), n = 9 (5xFAD PHP.eB-EGFP), n = 7 (5xFAD PHP.eB-circPOLR2A(9,10)). h, Probe trials assessed spatial memory retention. Overexpression of circPOLR2A(9,10) rescued memory deficits in 5xFAD mice. n = 11 (WT PHP.eB-EGFP), n = 9 (5xFAD PHP.eB-EGFP), n = 7 (5xFAD PHP.eB-circPOLR2A). i-j, Swim speed in MWM showed no significant difference. k, Rotarod test showed intact motor function across all groups. c-f, h and j-k, Mean values ± SEMs were shown. Data were analyzed by two-tailed Student’s t test. g and i, Mean values ± SEMs were shown, analyzed by two-way ANOVA.
Extended Data Fig. 10 AAV-mediated ds-cRNAs delivery alleviates microglia inflammation.
a, Microglial cells (CD11b+) in the brain of 5xFAD mice expressed over ten times higher levels of Il6 and Tnfα compared to other cells (CD11b−), as detected by RT-qPCR. n = 3. Mean values ± SEM were presented. b, Schematic illustrating the process of microglia isolation and RNA preparation for RNA-seq. c, Overflow of RNA-seq for microglia to identify differentially expressed genes (DEGs). The experimental groups included WT_EGFP (WT PHP.eB-EGFP, n = 3), AD_EGFP (5xFAD PHP.eB-EGFP, n = 2), AD_ds-cRNA (5xFAD PHP.eB-circPOLR2A, n = 4), and AD_PKR-KO (5xFAD; PKR-KO, n = 4). d-e, Venn diagram showed overlap of DEGs across experimental groups. Up-regulated genes (d) and down-regulated genes (e) in AD_EGFP vs. WT_EGFP and their overlap with other conditions. f, Gene Ontology (GO) analysis of 938 up-regulated genes in AD_EGFP vs. WT_EGFP comparison. The bar graph showed enriched biological processes, with immune response-related pathways being prominently represented. g, Heatmap showed that the expression of 69 up-regulated genes in AD_EGFP vs. WT_EGFP comparison was rescued with circPOLR2A delivery across experimental groups. h, Ds-cRNA reduced neuroinflammation in AD by inhibiting PKR overactivation, thereby decreasing toxic protein accumulation, protecting neurons, and rescuing memory deficits.
Supplementary information
Source data
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Feng, X., Jiang, BW., Zhai, SN. et al. Circular RNA aptamers targeting neuroinflammation ameliorate Alzheimer disease phenotypes in mouse models.
Nat Biotechnol (2025). https://doi.org/10.1038/s41587-025-02624-w
-
Received:
-
Accepted:
-
Published:
-
DOI: https://doi.org/10.1038/s41587-025-02624-w