Now Reading: Delivering base editors to the liver and lungs in alpha-1 antitrypsin deficiency
-
01
Delivering base editors to the liver and lungs in alpha-1 antitrypsin deficiency
Delivering base editors to the liver and lungs in alpha-1 antitrypsin deficiency
- Research Briefing
- Published:
(2025)Cite this article
Subjects
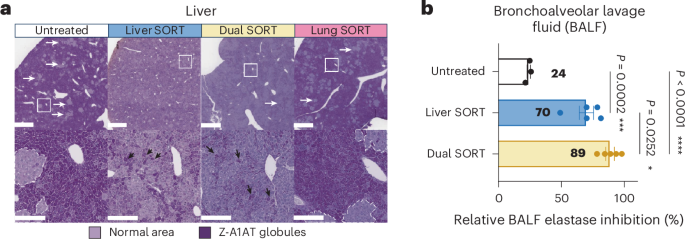
We developed Dual Selective Organ-Targeting Lipid Nanoparticles (Dual SORT LNPs) capable of delivering base editors to multiple organs. Base editor Dual SORT LNPs corrected disease-causing mutations in both the liver and lungs, the primary organs affected in alpha-1 antitrypsin deficiency.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
27,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
195,33 € per year
only 16,28 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

References
-
Strnad, P., McElvaney, N. G. & Lomas, D. A. Alpha1-antitrypsin deficiency. N. Engl. J. Med. 382, 1443–1455 (2020). A review article on AATD.
-
Zamora, M. R. & Ataya, A. Lung and liver transplantation in patients with alpha-1 antitrypsin deficiency. Ther. Adv. Chronic Dis. 12, (Suppl.) 20406223211002988 (2021). A review article that presents current treatment options for AATD.
-
Stiles, K. M. et al. Intrapleural gene therapy for alpha-1 antitrypsin deficiency-related lung disease. Chronic Obstr. Pulm. Dis. 5, 244–257 (2018). A review article on gene therapy for AATD.
-
Cheng, Q. et al. Selective organ targeting (SORT) nanoparticles for tissue-specific mRNA delivery and CRISPR-Cas gene editing. Nat. Nanotechnol. 15, 313–320 (2020). This paper reports the LNP SORT methodology.
-
Beam Therapeutics. A phase 1/2 dose-exploration and dose-expansion study to evaluate the safety and efficacy of BEAM-302 in adult patients with alpha-1 antitrypsin deficiency (AATD)-associated lung disease and/or liver disease. https://clinicaltrials.gov/study/NCT06389877 (2025). A clinical study that presents base editor AATD treatment.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This is a summary of: Kim, M. et al. Dual SORT LNPs for multi-organ base editing. Nat. Biotechnol. https://doi.org/10.1038/s41587-025-02675-z (2025).
Rights and permissions
About this article
Cite this article
Delivering base editors to the liver and lungs in alpha-1 antitrypsin deficiency.
Nat Biotechnol (2025). https://doi.org/10.1038/s41587-025-02705-w
-
Published:
-
DOI: https://doi.org/10.1038/s41587-025-02705-w