Now Reading: FINALLY Miracle Combo – Weight Loss and Muscle Growth Without Steroids
-
01
FINALLY Miracle Combo – Weight Loss and Muscle Growth Without Steroids
FINALLY Miracle Combo – Weight Loss and Muscle Growth Without Steroids
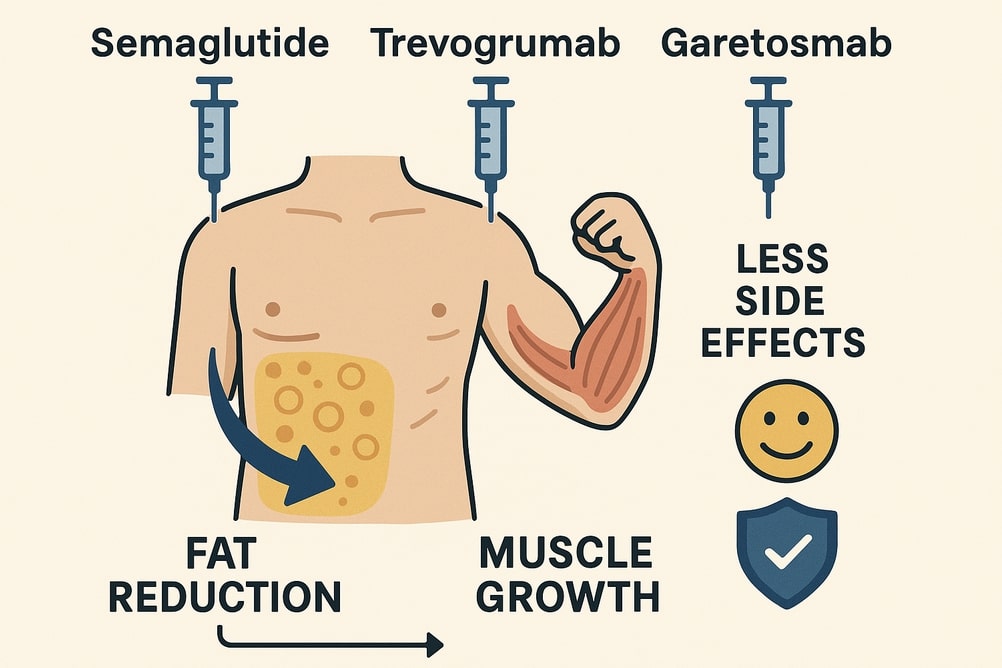
A combination treatment of semaglutide, trevogrumad and garetosmab causes fat loss and muscle growth in primate trials. Full trial results for humans are expected later in 2025. Results from the Phase II COURAGE trial show that Regeneron’s combination of semaglutide plus trevogrumab significantly enhanced fat loss while preserving lean muscle mass in patients with obesity.

Recent clinical research has explored the combination of semaglutide (a GLP-1 receptor agonist), trevogrumab (a myostatin inhibitor), and garetosmab (an activin A inhibitor) for obesity treatment, aiming to maximize fat loss while preserving or increasing lean muscle mass.


How They Work?
Semaglutide: Promotes weight loss by reducing appetite and caloric intake via GLP-1 receptor activation
Trevogrumab: Blocks myostatin, a protein that limits muscle growth, thereby promoting muscle mass increase
Garetosmab: Inhibits activin A, AB, and AC, which are also negative regulators of muscle mass, further supporting muscle growth and fat reduction
Clinical Trial Findings – Phase 2 COURAGE Trial
Design: Randomized, double-blind, with multiple arms including semaglutide alone, semaglutide plus trevogrumab (high/low dose), and semaglutide plus trevogrumab and garetosmab.
Duration: Two 26-week periods (weight loss and weight maintenance phases)

Results:
Fat Loss: Combination therapy led to greater fat loss than semaglutide alone.
Muscle Preservation: Patients on combination therapy preserved more lean mass; semaglutide alone resulted in 34.5% of weight loss from lean mass, while the combination reduced this proportion
Muscle Growth: In healthy participants, thigh muscle volume increased by 7.7% with trevogrumab 6 mg/kg plus garetosmab 10 mg/kg after a single dose, and fat mass decreased by up to 6.7%

Side Effects

The combination therapy, especially the triplet regimen, is associated with a higher incidence of side effects compared to semaglutide alone.
Notable side effects include:
Gastrointestinal Issues:
Common with GLP-1 receptor agonists like semaglutide, side effects such as nausea, vomiting, and diarrhea were reported across all groups. These are likely more pronounced in the combination arms due to the additive effects of the drugs.
Higher Discontinuation Rates:
The triplet therapy group had a 28.3% discontinuation rate due to tolerability issues and adverse events, significantly higher than the 4% to 10% seen in the semaglutide-only or semaglutide + trevogrumab groups.
Severe Adverse Events:
Severe treatment-emergent adverse events (TEAEs) occurred in 10.1% of the triplet therapy group, compared to 2% in the semaglutide-only group.
Two deaths were reported in the triplet group, though it’s unclear if they were treatment-related.
Unestablished Long-Term Safety:
As trevogrumab and garetosmab are experimental, their long-term safety profiles are not yet fully known, adding an element of uncertainty to the combination’s risk profile.
Interestingly, the combination of semaglutide and trevogrumab alone (without garetosmab) was better tolerated, with discontinuation rates similar to semaglutide monotherapy, suggesting that garetosmab may contribute to the increased side effects in the triplet regimen.

Brian Wang is a Futurist Thought Leader and a popular Science blogger with 1 million readers per month. His blog Nextbigfuture.com is ranked #1 Science News Blog. It covers many disruptive technology and trends including Space, Robotics, Artificial Intelligence, Medicine, Anti-aging Biotechnology, and Nanotechnology.
Known for identifying cutting edge technologies, he is currently a Co-Founder of a startup and fundraiser for high potential early-stage companies. He is the Head of Research for Allocations for deep technology investments and an Angel Investor at Space Angels.
A frequent speaker at corporations, he has been a TEDx speaker, a Singularity University speaker and guest at numerous interviews for radio and podcasts. He is open to public speaking and advising engagements.