Now Reading: Leveraging base excision repair for efficient adenine base editing of mitochondrial DNA
-
01
Leveraging base excision repair for efficient adenine base editing of mitochondrial DNA
Leveraging base excision repair for efficient adenine base editing of mitochondrial DNA
Data availability
Targeted deep sequencing data, whole-transcriptome sequencing data and whole-genome sequencing data can be accessed from the the National Center for Biotechnology Information under BioProjects PRJNA1223321, PRJNA1221787 and PRJNA1221509. SNV data from dbSNP (version 151; https://ncbi.nlm.nih.gov/snp/), integrated into the published BEIDOU toolkit, were used to identify de novo SNVs. Source data are provided with this paper.
Code availability
The custom Perl and Shell scripts for calculating frequencies of base substitution are available from GitHub (https://github.com/YangLab/CFBI).
References
-
Kim, J. S. & Chen, J. Base editing of organellar DNA with programmable deaminases. Nat. Rev. Mol. Cell Biol. 25, 34–45 (2024).
-
Barrera-Paez, J. D. & Moraes, C. T. Mitochondrial genome engineering coming-of-age. Trends Genet. 38, 869–880 (2022).
-
Silva-Pinheiro, P. & Minczuk, M. The potential of mitochondrial genome engineering. Nat. Rev. Genet. 23, 199–214 (2022).
-
Komor, A. C., Kim, Y. B., Packer, M. S., Zuris, J. A. & Liu, D. R. Programmable editing of a target base in genomic DNA without double-stranded DNA cleavage. Nature 533, 420–424 (2016).
-
Gaudelli, N. M. et al. Programmable base editing of A•T to G•C in genomic DNA without DNA cleavage. Nature 551, 464–471 (2017).
-
Li, X. et al. Base editing with a Cpf1–cytidine deaminase fusion. Nat. Biotechnol. 36, 324–327 (2018).
-
Wang, X. et al. Efficient base editing in methylated regions with a human APOBEC3A–Cas9 fusion. Nat. Biotechnol. 36, 946–949 (2018).
-
Yang, B., Yang, L. & Chen, J. Development and application of base editors. CRISPR J. 2, 91–104 (2019).
-
Yang, L. & Chen, J. A tale of two moieties: rapidly evolving CRISPR/Cas-based genome editing. Trends Biochem. Sci. 45, 874–888 (2020).
-
Richter, M. F. et al. Phage-assisted evolution of an adenine base editor with improved Cas domain compatibility and activity. Nat. Biotechnol. 38, 883–891 (2020).
-
Wang, X. et al. Cas12a base editors induce efficient and specific editing with low DNA damage response. Cell Rep. 31, 107723 (2020).
-
Wang, L. et al. Eliminating base-editor-induced genome-wide and transcriptome-wide off-target mutations. Nat. Cell Biol. 23, 552–563 (2021).
-
Han, W. et al. Design and application of the transformer base editor in mammalian cells and mice. Nat. Protoc. 18, 3194–3228 (2023).
-
Cho, S. I. et al. Targeted A-to-G base editing in human mitochondrial DNA with programmable deaminases. Cell 185, 1764–1776 (2022).
-
Mok, B. Y. et al. A bacterial cytidine deaminase toxin enables CRISPR-free mitochondrial base editing. Nature 583, 631–637 (2020).
-
Guo, J. et al. A DddA ortholog-based and transactivator-assisted nuclear and mitochondrial cytosine base editors with expanded target compatibility. Mol. Cell 83, 1710–1724 (2023).
-
Huang, J. et al. Discovery of deaminase functions by structure-based protein clustering. Cell 186, 3182–3195 (2023).
-
Mi, L. et al. DddA homolog search and engineering expand sequence compatibility of mitochondrial base editing. Nat. Commun. 14, 874 (2023).
-
Lim, K., Cho, S. I. & Kim, J. S. Nuclear and mitochondrial DNA editing in human cells with zinc finger deaminases. Nat. Commun. 13, 366 (2022).
-
Willis, J. C. W., Silva-Pinheiro, P., Widdup, L., Minczuk, M. & Liu, D. R. Compact zinc finger base editors that edit mitochondrial or nuclear DNA in vitro and in vivo. Nat. Commun. 13, 7204 (2022).
-
Sun, H. et al. Developing mitochondrial base editors with diverse context compatibility and high fidelity via saturated spacer library. Nat. Commun. 14, 6625 (2023).
-
Fauser, F. et al. Compact zinc finger architecture utilizing toxin-derived cytidine deaminases for highly efficient base editing in human cells. Nat. Commun. 15, 1181 (2024).
-
Yin, L., Shi, K. & Aihara, H. Structural basis of sequence-specific cytosine deamination by double-stranded DNA deaminase toxin DddA. Nat. Struct. Mol. Biol. 30, 1153–1159 (2023).
-
Krokan, H. E. & Bjoras, M. Base excision repair. Cold Spring Harb. Perspect. Biol. 5, a012583 (2013).
-
Beard, W. A., Horton, J. K., Prasad, R. & Wilson, S. H. Eukaryotic base excision repair: new approaches shine light on mechanism. Annu. Rev. Biochem. 88, 137–162 (2019).
-
Devchand, P. R., McGhee, J. D. & van de Sande, J. H. Uracil-DNA glycosylase as a probe for protein–DNA interactions. Nucleic Acids Res. 21, 3437–3443 (1993).
-
Carter, R. J. & Parsons, J. L. Base excision repair, a pathway regulated by posttranslational modifications. Mol. Cell. Biol. 36, 1426–1437 (2016).
-
Lei, L. et al. APOBEC3 induces mutations during repair of CRISPR–Cas9-generated DNA breaks. Nat. Struct. Mol. Biol. 25, 45–52 (2018).
-
Georgiadis, P., Polychronaki, N. & Kyrtopoulos, S. A. Progress in high-throughput assays of MGMT and APE1 activities in cell extracts. Mutat. Res. 736, 25–32 (2012).
-
Chen, J., Miller, B. F. & Furano, A. V. Repair of naturally occurring mismatches can induce mutations in flanking DNA. eLife 3, e02001 (2014).
-
Keijzers, G., Liu, D. & Rasmussen, L. J. Exonuclease 1 and its versatile roles in DNA repair. Crit. Rev. Biochem. Mol. Biol. 51, 440–451 (2016).
-
Cymerman, I. A., Chung, I., Beckmann, B. M., Bujnicki, J. M. & Meiss, G. EXOG, a novel paralog of endonuclease G in higher eukaryotes. Nucleic Acids Res. 36, 1369–1379 (2008).
-
Yang, C. et al. Structural insights into DNA degradation by human mitochondrial nuclease MGME1. Nucleic Acids Res. 46, 11075–11088 (2018).
-
Zheng, L. et al. Human DNA2 is a mitochondrial nuclease/helicase for efficient processing of DNA replication and repair intermediates. Mol. Cell 32, 325–336 (2008).
-
Mok, B. Y. et al. CRISPR-free base editors with enhanced activity and expanded targeting scope in mitochondrial and nuclear DNA. Nat. Biotechnol. 40, 1378–1387 (2022).
-
Mok, Y. G. et al. Base editing in human cells with monomeric DddA–TALE fusion deaminases. Nat. Commun. 13, 4038 (2022).
-
Yi, Z. et al. Strand-selective base editing of human mitochondrial DNA using mitoBEs. Nat. Biotechnol. 42, 498–509 (2024).
-
Hu, J. et al. Strand-preferred base editing of organellar and nuclear genomes using CyDENT. Nat. Biotechnol. 42, 936–945 (2024).
-
Cho, S. I. et al. Engineering TALE-linked deaminases to facilitate precision adenine base editing in mitochondrial DNA. Cell 187, 95–109 (2024).
-
Corona, P. et al. A novel mtDNA mutation in the ND5 subunit of complex I in two MELAS patients. Ann. Neurol. 49, 106–110 (2001).
-
Lebon, S. et al. Recurrent de novo mitochondrial DNA mutations in respiratory chain deficiency. J. Med. Genet. 40, 896–899 (2003).
-
Lei, Z. et al. Mitochondrial base editor induces substantial nuclear off-target mutations. Nature 606, 804–811 (2022).
-
Alexeyev, M., Shokolenko, I., Wilson, G. & LeDoux, S. The maintenance of mitochondrial DNA integrity–critical analysis and update. Cold Spring Harb. Perspect. Biol. 5, a012641 (2013).
-
Fontana, G. A. & Gahlon, H. L. Mechanisms of replication and repair in mitochondrial DNA deletion formation. Nucleic Acids Res. 48, 11244–11258 (2020).
-
Reddy, P. et al. Selective elimination of mitochondrial mutations in the germline by genome editing. Cell 161, 459–469 (2015).
-
Peeva, V. et al. Linear mitochondrial DNA is rapidly degraded by components of the replication machinery. Nat. Commun. 9, 1727 (2018).
-
Lapinaite, A. et al. DNA capture by a CRISPR–Cas9-guided adenine base editor. Science 369, 566–571 (2020).
-
Nilsen, H. et al. Uracil-DNA glycosylase (UNG)-deficient mice reveal a primary role of the enzyme during DNA replication. Mol. Cell 5, 1059–1065 (2000).
-
Raguram, A., Banskota, S. & Liu, D. R. Therapeutic in vivo delivery of gene editing agents. Cell 185, 2806–2827 (2022).
-
Yang, J. et al. ULtiMATE system for rapid assembly of customized TAL effectors. PLoS ONE 8, e75649 (2013).
-
Wang, L. et al. Enhanced base editing by co-expression of free uracil DNA glycosylase inhibitor. Cell Res. 27, 1289–1292 (2017).
-
McKenna, A. et al. The Genome Analysis Toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 20, 1297–1303 (2010).
-
Wilm, A. et al. LoFreq: a sequence-quality aware, ultra-sensitive variant caller for uncovering cell-population heterogeneity from high-throughput sequencing datasets. Nucleic Acids Res. 40, 11189–11201 (2012).
-
Kim, S. et al. Strelka2: fast and accurate calling of germline and somatic variants. Nat. Methods 15, 591–594 (2018).
-
Lee, S., Lee, H., Baek, G. & Kim, J. S. Precision mitochondrial DNA editing with high-fidelity DddA-derived base editors. Nat. Biotechnol. 41, 378–386 (2023).
Acknowledgements
This work is supported by the National Key Research and Development Program of China (2024YFA1803301 to J.C., 2024YFC3405902 to L.Y., 2023YFC3403402 to J.C. and 2023ZD0500501 to B.Y.), National Natural Science Foundation of China (32371514 to J.C., 32430018 to L.Y. and 32070170 to B.Y.), Ministry of Agriculture and Rural Affairs of the People’s Republic of China (NK2022010207 to J.C.), Shanghai Municipal Science and Technology Commission (23JS1400300 and 23DX1900102 to L.Y. and 23ZR1442500 to B.Y.) and Program of Shanghai Academic/Technology Research Leader (23XD1422500 to J.C.). We thank Shanghai Frontiers Science Center for Biomacromolecules and Precision Medicine, ShanghaiTech University and Molecular and Cell Biology Core Facility, School of Life Science and Technology, ShanghaiTech University for the technical support. We thank the Biomedical Big Data Platform at the Shanghai Institute for Advanced Immunochemical Studies, ShanghaiTech University for their deep sequencing services. FACS was provided by the Shanghai Institute for Advanced Immunochemical Studies, ShanghaiTech University, and we thank P. W. Zhang and L. S. Zhang for their assistance with cell sorting.
Ethics declarations
Competing interests
J.C., L.Y. and B.Y. are scientific cofounders of CorrectSequence Therapeutics, a company that uses gene-editing technologies, and members of its scientific advisory board. The other authors declare no competing interests.
Peer review
Peer review information
Nature Biotechnology thanks Hyongbum Kim, Bin Shen and Yangming Wang for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 TALED, but not DdCBE, induced bystander editing in TALE-binding regions at other target sites.
a, Schematic of the constructions of sTALED and DdCBE targeting ND4 site. NTD, N-terminal domain of TALE array; CTD, C-terminal domain of TALE array; MTS, mitochondrial targeting sequence; UGI, uracil glycosylase inhibitor. b, A-to-G editing frequencies induced by sTALED and C-to-T base editing frequencies induced by DdCBE at the ND4 site in 293FT cells. NT, non-transfected control. c, Schematic of the constructions of sTALED and DdCBE targeting ND5.3 site. d, A-to-G editing frequencies induced by sTALED and C-to-T base editing frequencies induced by DdCBE at the ND5.3 site in 293FT cells. The sequences of TALE-binding regions are in blue and bystander editing can be found in the boxes with dashed line. The data are presented as the mean ± s.d. from three independent experiments.
Extended Data Fig. 2 Purification of TALED/DdCBE-related proteins.
a, Size-exclusion chromatography profile of the His-tagged L_TALE-DddA_1397N. The indicated peak fraction was analyzed by SDS-PAGE. b, Size-exclusion chromatography profile of the His-tagged R_TALE-DddA_1397C. The indicated peak fraction was analyzed by SDS-PAGE. c, Size-exclusion chromatography profile of the His-Sumo-tagged R_TALE-DddA_1397C-TadA8e. The indicated peak fraction was analyzed by SDS-PAGE. For a–c, the gels are representative of three independent experiments.
Extended Data Fig. 3 In vitro adenosine deamination assay for TALEDs.
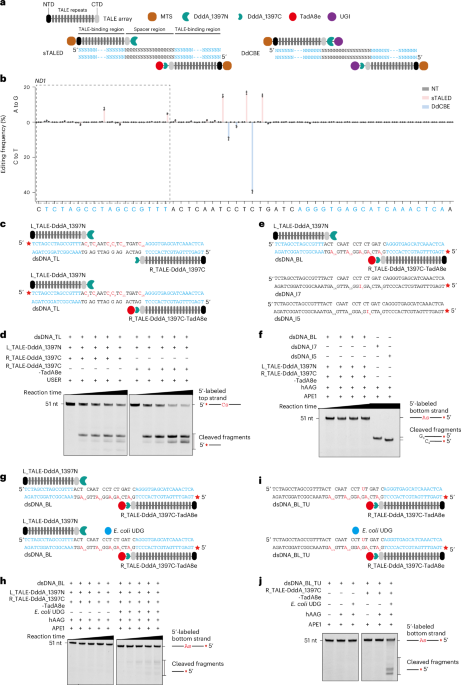
a, Schematic of the constructions of R_TALE-DddA_1397C-TadA8e and the synthesized ssDNA that contains only one adenosine at position 11 (ssDNA_A11). b, In vitro adenosine deamination assay of R_TALE-DddA_1397C-TadA8e with ssDNA_A11 as the substrate. c, Schematic of the purified R_TALE-DddA_1397C-TadA8e targeting the synthesized ND1 dsDNA with the labeled bottom-strand and the top-strand that contains a tetrahydrofuran (dsDNA_BL_T#) in the absence or presence of APE1. d, In vitro adenosine deamination assay of R_TALE-DddA_1397C-TadA8e with dsDNA_BL_T# as the substrate. e, Schematic of the purified R_TALE-DddA_1397C-TadA8e targeting the synthesized ND1 dsDNA with the labeled bottom-strand that contains a single adenosine in spacer region and the top-strand that contains a fixed uridine (dsDNA_A2_BL_TU and dsDNA_A5_BL_TU). f, In vitro adenosine deamination assay of R_TALE-DddA_1397C-TadA8e with dsDNA_A2_BL_TU or dsDNA_A5_BL_TU as the substrate. The gels are representative of three independent experiments.
Extended Data Fig. 4 Roles of different DNA exonucleases in TALED-mediated A-to-G editing in mtDNA.
a, C-to-T editing frequencies induced by DdCBE at the ND1 and ND4 sites in wild-type (WT) and hUNG/hSMUG1 double-knockout (hUNG/hSMUG1_DKO) 293FT cells. b, A-to-G editing frequencies induced by sTALED at the ND1 and ND4 sites in WT and hAPE1 knockout (hAPE1_KO) 293FT cells. c, A-to-G editing frequencies induced by sTALED at the ND1 and ND4 sites in WT, hExo1 knockout (hExo1_KO), hExoG knockout (hExoG_KO) and hDNA2 knockout (hDNA2_KO) 293FT cells. d, Schematic diagram of the scenario in which hMGME1-mediated DNA strand excision destroys the binding region for TadA8e-containing half of sTALED and therefore no A-to-G editing is induced. e, Schematic diagram of the scenario in which DddA mediates C-to-U editing at both DNA strands in the spacer region and therefore DNA double strand breaks (DSBs) are generated. f, Schematic diagram of the scenario in which the previous round of A-to-G editing (red base pair) occurred in one DNA strand (dark green strand) within the spacer region and therefore a new round of A-to-G editing (orange base) can occur in the other DNA strand (light green strand). For a–c, the data are presented as the mean ± s.d. from three independent experiments.
Extended Data Fig. 5 Generation of hExoG, hMGME1 and hDNA2 knockout cells and overexpression of hUNG and hMGME1.
a, c, e, The knockouts of indicated genes were confirmed by sequencing the genomic loci. b, d, f, The effects of knocking out hExoG, hMGME1 and hDNA2 on protein expression were examined by Western blots. The blots for anti-hExoG/anti-hMGME1/anti-hDNA2 and anti-actin/anti-tubulin were performed with the same samples but run in different gels. g, The expression of myc-hUNG when editing the ND1 and ND4 sites in hUNG/hSMUG1_DKO cells was examined by Western blots. The blots for anti-myc and anti-tubulin were performed with the same samples but run in different gels. h, A-to-G editing frequencies induced by sTALED without or with myc-hUNG coexpression at the ND1 and ND4 sites in hUNG/hSMUG1_DKO cells. i, The expression of myc-hMGME1 when editing the ND1 and ND4 sites in hMGME1_KO cells was examined by Western blots. The blots for anti-myc and anti-tubulin were performed with the same samples but run in different gels. j, A-to-G editing frequencies induced by sTALED without or with myc-hMGME1 coexpression at the ND1 and ND4 sites in hMGME1_KO cells. For h and j, the data are presented as the mean ± s.d. from three independent experiments.
Extended Data Fig. 6 Comparison of the A-to-G editing and C-to-T editing induced by the TALEDs and DdCBEs with same TALE arrays.
a, Schematic of the constructions of sTALED and DdCBE targeting mitochondrial DNA. b, A-to-G and C-to-T editing frequencies induced by DdCBE and sTALED at the ND5.1, ND6.1, TRNG, ND4L and ND5.3 sites in 293FT cells. The data are presented as the mean ± s.d. from three independent experiments.
Extended Data Fig. 7 Editing efficiency induced by engineered TALEDs.
a, Schematic of the constructions of sTALED, sTALED6 and sTALED11 targeting mtDNA. b, A-to-G editing frequencies induced by sTALED, sTALED6 and sTALED11 at the ND5.1 and ND5.3 sites in 293FT cells. c, Schematic of the constructions of sTALED, esTALED_V2 and esTALED_V2_dhUNG targeting mtDNA. dhUNG, catalytically dead hUNG. d, A-to-G editing frequencies induced by sTALED, esTALED_V2 and esTALED_V2_dhUNG at the ND1 and ND5.3 sites in 293FT cells. e, Schematic of the constructions of sTALED_G1333 and esTALED_G1333 targeting mtDNA. f, A-to-G editing frequencies induced by sTALED_G1333 and esTALED_G1333 at the ND1, ND5.3 and ND5.3 sites in 293FT cells. For b, d and f, the data are presented as the mean ± s.d. from three independent experiments.
Extended Data Fig. 8 Comparison of editing efficiencies of mTALEDs and the effect of complete DddA deamination disruption on TALED efficiency.
a, Schematic of the constructions of mTALED, mTALED6_GSVG, emTALED6_GSVG_V1 and emTALED6_GSVG_V2 targeting mtDNA. b, A-to-G editing frequencies induced by mTALED, mTALED6_GSVG, emTALED6_GSVG_V1 and emTALED6_GSVG_V2 at the ND1 and ND5.1 sites in 293FT cells. c, Schematic of the constructions of mDdCBE_E1347A and mDdCBE_E1347A-G1348S targeting mtDNA. d, C-to-T editing frequencies induced by mDdCBE_E1347A and mDdCBE_E1347A-G1348S at the ND1, ND5.1 and ND5.3 sites in 293FT cells. e, Schematic of the constructions of dTALED and dTALED_E1347A-G1348S targeting mtDNA. f, A-to-G editing frequencies induced by dTALED and dTALED_E1347A-G1348S at the ND1, ND5.1 and ND5.3 sites in 293FT cells. For b, d and f, the data are presented as the mean ± s.d. from three independent experiments.
Extended Data Fig. 9 Comparison of edTALED6_GSVG with other mtDNA editors and characterization of its cell applicability and mtDNA-wide off-target editing.
a, Schematic of the construction of the dTALED, mitoABENt.BspD6I(C), monomeric mitoABENt.BspD6I(C) and edTALED6_GSVG targeting mtDNA. b, Heatmaps of A-to-G editing frequencies induced by the indicated editors. c and d, A-to-G editing frequencies induced by dTALED6_GSVG and edTALED6_GSVG in A549 (c) and U2OS (d) cells. For c and d, the data are presented as the mean ± s.d. from three independent experiments. e, The position and frequency of single base substitution in mtDNA induced by the indicated editors targeting the ND5.1 site, with the editing within the spacer region shown in red. The data are presented from three independent experiments.
Extended Data Fig. 10 Characterization of the editing efficiency, nuclear genome-wide mutation and cellular toxicity induced by engineered TALEDs.
a, Schematic of the constructions of sTALED, esTALED6 and esTALED6R targeting mtDNA. b, Heatmaps of A-to-G editing frequencies induced by the indicated editors. The sequences of the TALE-binding regions are in blue, and bystander editing can be found in the boxes with dashed black lines. The boxes with dashed blue lines represent the narrowed editing windows. c and d, Number of A/T-to-G/C (c) and C/G-to-T/A (d) nuclear genome-wide substitutions induced by the indicated editors. e and f, Cell viability was measured by MTS assay at day 2 (e) and day 4 (f) post-transfection after treated with the indicated editors targeting ND1 site. For b, e and f, the data are presented as the mean ± s.d. from three independent experiments. For c and d, the eGFP data are presented as the mean ± s.d. from two independent experiments, sTALED, esTALED6 and esTALED6R are presented as the mean ± s.d. from three independent experiments. Statistical analysis was performed via two-tailed Student’s t-test. For e and f, statistical analysis was performed via two-tailed Welch’s t-test.
Supplementary information
Source data
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fan, Y., Xu, W., Gao, BQ. et al. Leveraging base excision repair for efficient adenine base editing of mitochondrial DNA.
Nat Biotechnol (2025). https://doi.org/10.1038/s41587-025-02608-w
-
Received:
-
Accepted:
-
Published:
-
DOI: https://doi.org/10.1038/s41587-025-02608-w