Now Reading: New Breakthrough: Natural Solution Promises Weight Loss and Muscle Growth
-
01
New Breakthrough: Natural Solution Promises Weight Loss and Muscle Growth
New Breakthrough: Natural Solution Promises Weight Loss and Muscle Growth
Fast Summary
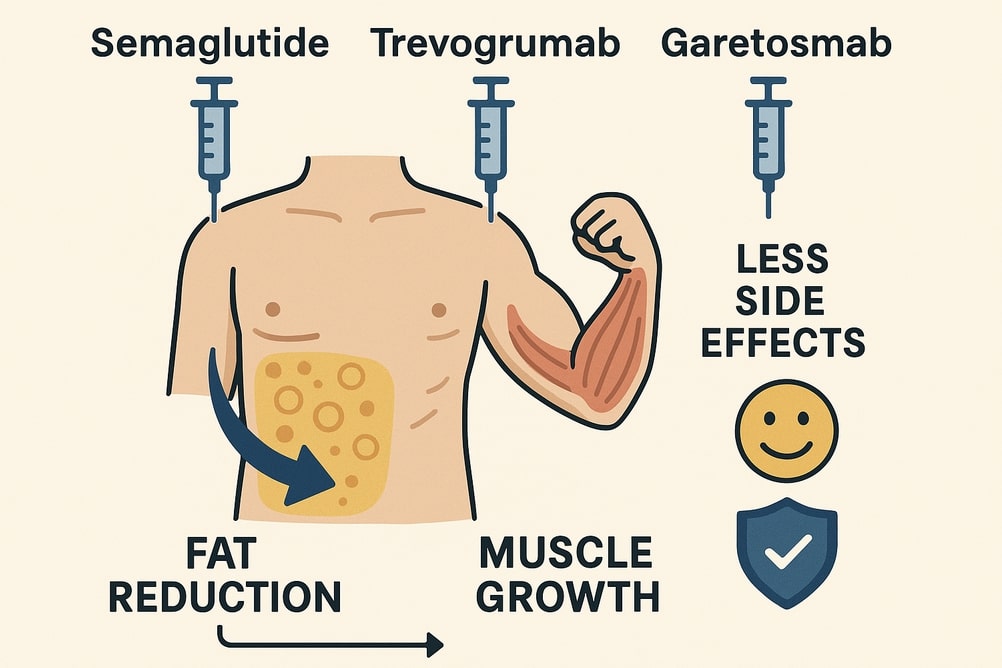
- A new combination treatment involving semaglutide, trevogrumab, and garetosmab has demonstrated increased fat loss and muscle growth in primates during clinical trials, with human trials expected to conclude in 2025.
- Phase II COURAGE trial findings:
– Combination therapy using semaglutide,trevogrumab,and garetosmab resulted in important fat loss while preserving lean muscle mass.
– Muscle volume increased by up to 7.7%, while fat mass decreased by up to 6.7% after a single dose of the triplet regimen in healthy participants.
- Mechanisms of action:
– Semaglutide reduces appetite through GLP-1 receptor activation, aiding weight loss.
– Trevogrumab blocks myostatin to encourage muscle growth.
– Garetosmab inhibits activin A/AB/AC for further muscle enhancement and fat reduction.
- Side effects include:
– High rates of gastrointestinal issues like nausea and vomiting due to additive drug effects.
– Increased discontinuation rates (28.3%) for the triplet therapy due to tolerability concerns; deaths were reported but unclear if connected directly to treatment.- Severe adverse events occurred significantly more frequently enough with triplet therapy compared to other arms (10% vs. ~2%). Long-term safety is uncertain.
Indian Opinion Analysis
The potential breakthrough achieved through this experimental combination therapy reflects promising advancements in tackling obesity-a public health issue that increasingly affects populations worldwide, including India’s urban middle class grappling with lifestyle-related diseases. While the triple-drug regimen highlights innovative pharmacological solutions combining weight reduction with preservation or enhancement of lean muscle mass, risks associated with significant side effects need careful evaluation before widespread application.
For India specifically,where managing chronic conditions like obesity intersects with limited healthcare access in rural areas and affordability challenges among lower-income groups,such therapies may only benefit niche populations initially. Ensuring both efficacy and manageable side-effect profiles will be essential before rollout on a larger scale becomes feasible within an already overburdened healthcare system.
Given India’s rapid adoption rate for medical innovations when cost-effective options arise-akin to generics or biosimilars-human trial outcomes could pave pathways for stronger pharmacological partnerships or domestic production capabilities catering uniquely toward Indian needs.