Historically, treatment for blood cancers, relied heavily on chemotherapy. However, this “carpet bombing” approach, apart from destroying cancer cells also affects healthy, rapidly-dividing cells. This can lead to significant side effects like vomiting ,immunosuppression, hair loss and anaemia. While chemotherapy remains vital, the goal has always been more precise, less toxic options.
A deeper understanding of cancer cell biology has paved the way for “targeted therapy”—drugs designed as “surgical strikes” against molecular targets predominantly found on or within cancer cells. This approach aims for greater efficacy with a more manageable side-effect profile, sparing normal cells to a larger extent.
Imatinib – the dawn of targeted therapy in haematology
A pivotal moment in targeted therapy arrived with Imatinib, approved in 2001 for Chronic Myeloid Leukaemia (CML). CML is driven by the Philadelphia chromosome, a genetic abnormality creating the BCR-ABL fusion gene that fuels uncontrolled white blood cell proliferation. Before Imatinib, CML was often fatal within years.
Imatinib was designed to block the activity of this BCR-ABL protein. Its success was revolutionary, transforming CML from a deadly disease into a manageable chronic condition for most patients, who could now achieve long-term remission and a good quality of life with a daily pill. This validated the concept of molecularly targeted therapy and spurred massive research into similar approaches for other cancers, heralding a new frontier in cancer treatment .
Harnessing the immune system –the rise of immunotherapy in haematology
Our immune system, with its innate (first-line, non-specific) and adaptive (specialised, memory-forming) arms, is designed to eliminate threats, including cancerous cells. Immunotherapy aims to boost or re-engage the patient’s immune system to fight cancer. Various types of antibody based therapies such as Monoclonal Antibodies (mAbs) antibodies target specific antigens on cancer cells.
Bispecific Antibodies (e.g., BiTEs – Bispecific T-cell Engagers)have two binding sites– one for a cancer cell antigen and another for a T-cell (an immune killer cell). Antibody-Drug Conjugates (ADCs) are “armed antibodies” and combine an antibody’s targeting precision with a potent cytotoxic drug .
These immunotherapies offer highly effective options ,which earlier approved for resistant cancers ,are being increasingly used in frontline settings.
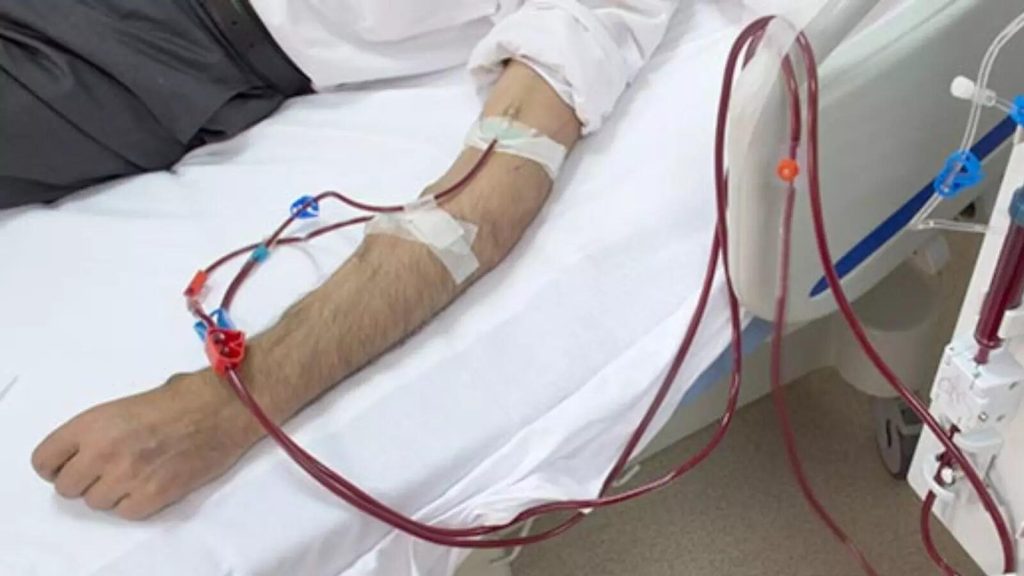
For thousands battling life-threatening blood cancers and other devastating diseases, a Bone Marrow Transplant (BMT) offers a beacon of hope – a potential cure when other treatments have failed. This remarkable medical procedure involves replacing a patient’s diseased or damaged bone marrow with healthy stem cells from the patient (autologous BMT) or a healthy donor (allogenic BMT) .
Recent advances in BMT
BMT is a critical treatment option for a range of blood conditions. These include benign conditions such as sickle cell anaemia, thalassemia, aplastic anaemia, inherited immune deficiency and metabolic disorders as well as malignant conditions like high risk acute leukemias, myelodysplastic syndrome, relapsed leukemias, myelomas and relapsed /refractory lymphomas.
Earlier, allogenic bone marrow transplant were done only with HLA matched donors (related or unrelated) .The probability of finding a matched donor was only 30%.
Now however, haploidentical (Haplo) BMT, a groundbreaking approach, allows for transplants using donors (typically family members like parents or children) who are only a half-match for the patient’s HLA type.This involves the use of PTCY – Post transplant cyclophosphamide, where chemotherapy is administered on day +3 and day +4 after infusion of the stem cells. This has dramatically increased donor availability, meaning most patients who need a transplant can now find a suitable donor.
Traditionally, BMT involved high-dose chemotherapy and/or radiation (myeloablative conditioning) to wipe out the patient’s marrow. Reduced Intensity Conditioning (RIC) Transplant regimens use lower, less toxic doses, making transplants an option for older patients or those with other health conditions who might not tolerate aggressive conditioning. This relies more on the graft versus leukemia effect, where the donor cells act against the cancer of the recipient .
Improved Graft Manipulation: Scientists area also developing sophisticated ways to process donor stem cells before infusion such as TCR α/β depletion which removes specific T-cells (T-cell receptor alpha/beta cells) from the donor graft that are primarily responsible for causing graft versus host disease (GVHD).
With ongoing research and innovation, the future promises even better outcomes, reduced side effects, and wider applicability, offering a renewed lease on life to countless individuals around the world.
CAR T-cell therapy: living drugs to combat cancer
Chimeric Antigen Receptor (CAR) T-cell therapy is a groundbreaking immunotherapy that engineers a patient’s own T-cells into potent cancer-killing “living drugs.” The process involves collecting a patient’s T-cells, genetically modifying them in the lab to express a CAR that recognises a specific antigen on their cancer cells (e.g., CD19 on B-cell leukaemias/lymphomas), expanding these engineered cells, and then infusing them back into the patient.
Once infused, CAR T-cells seek out and destroy cancer cells expressing the target antigen. This therapy has achieved unprecedented success in patients with relapsed or refractory B-cell acute lymphoblastic leukaemia and certain non-Hodgkin lymphomas, leading to high remission rates and several FDA-approved products. However, challenges include significant potential side effects like Cytokine Release Syndrome (CRS) and neurotoxicity (ICANS), along with high manufacturing complexity and cost.
Gene therapy, AI and the importance of foundational clinical skills
Gene therapy offers curative potential for inherited haematological disorders by modifying a patient’s cells, often by introducing a functional gene or correcting a faulty one. Haematopoietic stem cells (HSCs) from bone marrow are prime targets, as modified HSCs can repopulate the marrow with corrected cells, offering a permanent solution. Significant progress has been made in diseases such as sickle cell disease, Beta-thalassemia and Hemophilia A in this regard. While transformative, challenges like long-term durability, cost, and access remain.
Artificial Intelligence (AI) is emerging as a powerful tool in haematology, capable of analysing vast and complex datasets to enhance diagnosis, treatment, and research. Key applications include:
AI-powered diagnostics: AI algorithms analyse blood smears and bone marrow biopsies to identify and classify cells with high accuracy, potentially assisting pathologists and improving diagnostic efficiency. AI also helps interpret complex genomic data from NGS.
Accelerating drug discovery: AI can identify novel therapeutic targets, predict drug efficacy and toxicity, and repurpose existing drugs for haematological conditions, streamlining development. It can also aid in patient stratification for trials and accelerate data analysis, potentially speeding up drug approvals. AI can also integrate diverse patient data to predict individual responses to therapies, helping clinicians choose optimal treatment plans. It is poised to act as an “AI physician assistant,” augmenting human expertise rather than replacing it, leading to more precise and personalised care.
In this era of remarkable technological progress, from gene editing to AI, it’s vital to remember that the foundations of good medical practice such as thorough history taking, comprehensive physical examinations and focussed investigations are paramount in arriving at the right diagnosis. The future of haematology lies in the synergistic integration of traditional medical wisdom and cutting-edge technology, ensuring holistic patient care and continued progress against blood disorders.
This is the second story of the two-part series. You can read the first story here.
(Dr. Steve Thomas, is a clinical haemato-oncologist and BMT physician at Sri Ramachandra Medical College, Porur, Chennai. stev07thomas@sriramachandra.edu.in)
Published – June 15, 2025 07:30 am IST