Now Reading: Precision gene editing medicine makes history, and it’s just getting started
-
01
Precision gene editing medicine makes history, and it’s just getting started
Precision gene editing medicine makes history, and it’s just getting started
Despite their astounding success, custom-made base editors and prime editors will need time to broaden their clinical impact.
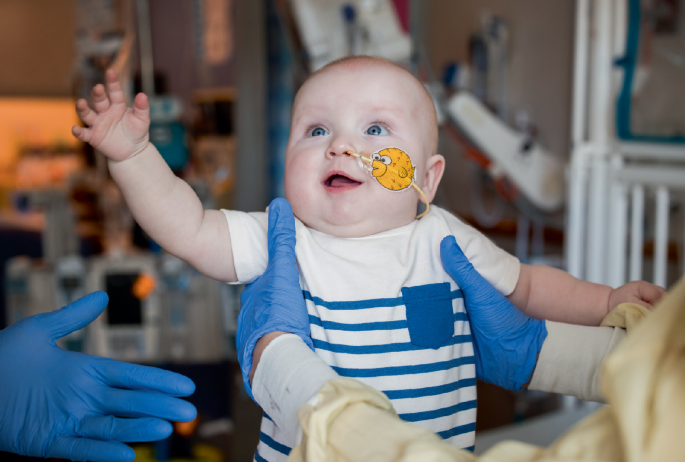
As in something out of science fiction, a team of researchers reached into the genome of a seven-month-old baby with a base editing therapy targeted specifically to repair his unique mutation. Time was of the essence, as the rare, life-threatening genetic disorder caused by a mutation in his carbamoyl phosphate synthetase 1 (CPS1) gene impairs the body’s ability to process protein, causing a toxic build-up of ammonia in the blood, affecting brain function. Half the babies with this urea cycle disorder die before their first birthday, and even when they survive, they experience severe developmental delays. The speed was remarkable: it took less than seven months to go from having the sequence in hand to delivering this first treatment of its kind, made just for him. Now nine month old, baby KJ, as he is known, appears healthy.

Baby KJ, born with a rare urea cycle disorder, was treated with the world’s first personalized gene editing therapy.
Credit: Children’s Hospital of Philadelphia
This “beautiful success story,” says David Liu of the Broad Institute, Harvard University and the Howard Hughes Medical Institute, who invented base and prime editors, has been decades in the making. The intervention was carried out at Children’s Hospital of Philadelphia (Box 1), but it took no less than 11 teams from both academia and industry to design the editing agents, engineer delivery vehicles, manufacture, perform safety testing and gain regulatory approval. “Each component took years, but now they came together [in a way] that allows the field to do this kind of intervention on a time scale that would seem unimaginably fast,” says Liu.
While baby KJ made news around the globe, the latest generations of gene editing medicines garnered fewer headlines but made equally impressive strides. In March, Beam Therapeutics released the results of a phase 1/2 trial of in vivo base editing medicine for α-1 antitrypsin deficiency. In April, Verve Therapeutics released dramatic cholesterol-lowering clinical results with their in vivo base editing treatment for familial hypercholesterolemia. And in May, Prime Medicine announced that the first patient to receive their prime editing medicine, an 18-year-old man with chronic granulomatosis disease, saw his health improve rapidly after a single injection of the therapy.
Not to be forgotten, the original CRISPR–Cas9 therapies also made some news. Intellia Therapeutics’ nexiguran ziclumeran (nex-z) — the first systemic gene editing therapy in humans — entered phase 3 trials. This treatment, for hereditary transthyretin amyloidosis, reduced serum transthyretin by 90% at 28 days in phase 1 studies.
Trials with traditional CRISPR therapies still outnumber base and prime editors, but the new kids on the block are gaining ground. As of June, at least 20 clinical trials with base or prime editors are underway, and 9 have released some results (Table 1). The main distinction is in the enzymes: the newer base and prime editing medicines combine a CRISPR–Cas targeting agent with enzymes that modify or insert specific nucleotides into the DNA without creating double-stranded breaks. As such, these editors are more precise than CRISPR–Cas9 technologies and minimize the risk of mayhem caused by endogenous repair mechanisms recruited to fix double-stranded breaks.
CRISPR–Cas9-based medicines are likely to remain confined to what the tool does best: knock out unwanted or toxic genes. In contrast, base editing can be used to correct mutated genes by performing chemistry changes on single, targeted nucleotides. Base editors use a modified Cas9 protein known as a ‘nickase’ that creates nicks or breaks in a single strand of DNA, combined with a deaminase that acts at that site to remove an amino group from a base, turning cytosine to uracil, or adenine to inosine. Prime editors can rewrite a segment of DNA and replace the original sequence by using a reverse transcriptase in collaboration with a cellular repair mechanism (search and replace editing).
Base editing first entered the clinic in 2022, when a 13-year-old girl with relapsed T cell acute lymphoblastic leukemia was treated with base-edited CAR-T cells at Great Ormond Street Hospital in London. A group led by Waseem Qasim created off-the-shelf donor CAR-T cells edited in the lab to specifically inactivate the leukemic cells. With a CRISPR-guided cytidine deaminase editor, they targeted and inactivated three genes on the donors’ T cells: CD52, CD7 receptors, and a subunit of the T cell receptor. With these edits the engineered CAR-T cells could do their job without causing graft-versus-host-disease, or fighting each other in so-called T cell fratricide, while evading strong lymphodepleting effects of anti-CD52 serotherapy.
The child, Alyssa Tapley, now 16, has received accolades for undertaking this pioneering journey and, fittingly, in April presented the inventor of base editing, David Liu — whose work saved her life — with Silicon Valley’s 2025 Breakthrough Prize.
Meanwhile, Liu’s company Beam Therapeutics, which he founded with Keith Joung and Feng Zhang in 2017, late last year released clinical data from its first base-editing therapy for sickle cell disease. The therapy Beam-101 is an ex vivo, autologous hematopoietic stem cell, base edited to introduce mutations at position –175 of the hemoglobin promoter. This edit unblocks fetal hemoglobin by disabling a repressor gene. Thus liberated, fetal hemoglobin resumes production in adult erythroid cells. The company reports that in 17 treated patients, sickle cells were nearly completely eliminated, with only a few percent of red cells expressing sickling hemoglobin at six months.
Another first in this rapidly evolving journey is Verve Therapeutics’ in vivo base editing clinical trial for hypercholesterolemia. VERVE-101 is the first base editor being trialed inside the body. It employs (via an exclusive license) one of Beam’s adenosine editors to inactivate a hyperactive version of PCSK9, which encodes a protein (proprotein convertase subtilisin/kexin type 9) that prevents the uptake of low-density lipoprotein cholesterol (LDL-C) into cells, resulting in elevated plasma LDL-C. The base editing therapy is delivered with a proprietary N-acetylglucosamine (GlcNAc) lipid nanoparticle (LNP) that travels to the liver. Early trial results with the first version of this therapy were reported in late 2023 with positive results. The trial stopped enrolling patients, however, because of a single adverse event, which was alleviated by modifying the LNP. That also improved uptake in the liver.
The second-generation therapy, VERVE-102, led to up to 60% dose-dependent reductions in PCSK9 protein levels and a 53% mean drop in LDL-C levels in the four patients receiving the highest dose. According to Sekar Kathiresan, Verve’s co-founder and CEO, whose decades of work on the genetics of heart disease was the foundation for Verve, so far 13 patients have been treated with VERVE-101, and 14 patients with VERVE-102. The first cohort treated with VERVE-101 has experienced enduring effects after a single injection for as long as two years.
Shanghai based YolTech Therapeutics also recently reported results from an in vivo base editing investigator-initiated phase 1/2 clinical trial with its proprietary base editor, hpABE5, and LNP. Six patients with familial hypercholesterolemia have been treated so far, and the results are similar to those with Beam-302, with enduring reductions in plasma PCSK9 and LDL-C for as long as 36 weeks.
In vivo base editing has also been used to correct, rather than eliminate, a disease-causing mutation. Beam Therapeutics’ in vivo base editing therapy Beam-302 demonstrated for the first time that this was possible, in patients with α-1 antitrypsin deficiency. Beam-302, an adenosine base editor delivered in an LNP, corrects the ‘Piz’ mutation in the SERPINA1 gene, which encodes α-1 antitrypsin. When mutated, the α-1 antitrypsin protein made in the liver misfolds and aggregates, and lung and liver damage ensues. By making an A-to-G transition at position 342, the editor changes a lysine residue to glutamic acid. After a single injection of the editor in nine patients, the mutated protein fell by up to 78% from baseline at the highest dose and normal α-1 antitrypsin rose in a dose-dependent fashion.
Another milestone is Prime Medicine’s positive clinical trial results from the first prime editing medicine given to a person, PM359. The therapy, ex vivo edited autologous stem cells, was developed to treat chronic granulomatosis disease, which arises from mutations in the NADPH oxidase complex. People with mutations in any of the subunits that form the complex are unable to fight pathogens, leading to recurrent and intractable infections. Prime editor PM359 corrects the delGT mutation in NCF1 gene, which encodes p47phox, the most commonly occurring defect in autosomal recessive chronic granulomatosis disease. A month after a single infusion of PM359, an 18-year-old patient had NADPH oxidase activity restored in 66% of his neutrophils, well above the minimum of 20% predicted to be needed for positive clinical outcome. Another plus is that engraftment of the transplanted cells was faster than that in approved gene editing technologies. According to Mohammed Asmal, chief medical officer of Prime Medicine, this may be because prime editing is less toxic to cells and may cause less damage to the DNA than CRISPR–Cas9. “Because prime editing doesn’t induce double-strand break in the DNA, prime editing is better tolerated. The cells likely recover faster after infusion so they replicate more quickly and you get faster engraftment,” says Asmal. In a recent study from Carl June’s lab directly comparing the properties of CAR-T cells engineered with CRISPR–Cas9 with those using an adenosine base editor, the base edited cells outperformed those created with CRISPR–Cas 9 in multiple aspects, including manufacturing yields and tumor control and survival in a mouse leukemia model.
Impressive as the trial results are — all positive and enduring with no strong safety signals so far — some issues remain, among them moving beyond the few tractable organ systems (stem cells and liver) and scaling up. To date only roughly 50 people have received any of the base or prime editing treatments, estimates Liu. “We will need a new model — whether that involves federal, philanthropic, academic, industrial, or most likely a combination of all of the above — to connect this rapidly evolving and increasingly capable science to those patients who most urgently need these treatments,” he says (Box 1).
Some of the solutions are in the science itself, say Liu. “We can use our creativity to apply base and prime editors and other gene editing techniques in ways that offer benefits to larger swaths of patients.” His teams are developing editors that work in mutation-agnostic ways, so that regardless of the mutation, you can derive benefit from one drug, one composition of matter. This could streamline regulatory requirements by de-risking certain features. “The question is at what point […] might a regulatory body say: ‘We believe these agents do what they say they do’,” he says.
Many are indeed optimistic that the path to the clinic for base and prime editing medicines will hasten, especially thanks to gene editing medicines that work in vivo. These systemic therapies are not encumbered by the scaling challenges of ex vivo treatments: the time and expense of removing cells from the patient, treating and growing them, as well as patient care throughout. “It’s more of a synthetic chemistry process,” says Prime Medicine’s Asmal. “This will allow costs to drop dramatically.
As with all new technologies, however, the next generation of editing technologies will need the blessing of the US Food and Drug Administration (FDA). At the agency, despite the changes at the top, it’s business as usual, says Verve’s Kathiresan. “It took a couple of years for them to get their head around all this, but I think they have now. They have a team that really understands what we’re trying to do,” says Kathiresan. In the last year and a half, the FDA cleared a number of Investigational New Drug applications for in vivo gene editing, including Verve’s for hypercholesterolemia (VERVE-102).
Genetic diseases will always be part of humanity because they are a fundamental consequence of the chemical structure of our DNA, which is not going to change, says Liu. “It is a massive health problem, but it often flies under that radar because hundreds of millions of patients are subdivided into thousands of different genetic disease communities; it make economics challenging for industry.” Next-generation editing agents, Liu foresees, will “find ways to help take control of our genome so we are not beholden to our misspellings in our DNA.”