Now Reading: TransEuro Trial Explores Fetal Cell Transplantation for Parkinson’s Treatment
-
01
TransEuro Trial Explores Fetal Cell Transplantation for Parkinson’s Treatment
TransEuro Trial Explores Fetal Cell Transplantation for Parkinson’s Treatment
Quick Summary
- Neural transplantation for Parkinson’s disease (PD), specifically using human fetal ventral mesencephalon (hfVM) tissue, has been explored to replace lost dopaminergic neurons critical to PD pathology.
- Previous trials yielded inconsistent results: some patients showed long-term motor improvements, while others experienced disabling side effects like graft-induced dyskinesias (GIDs).
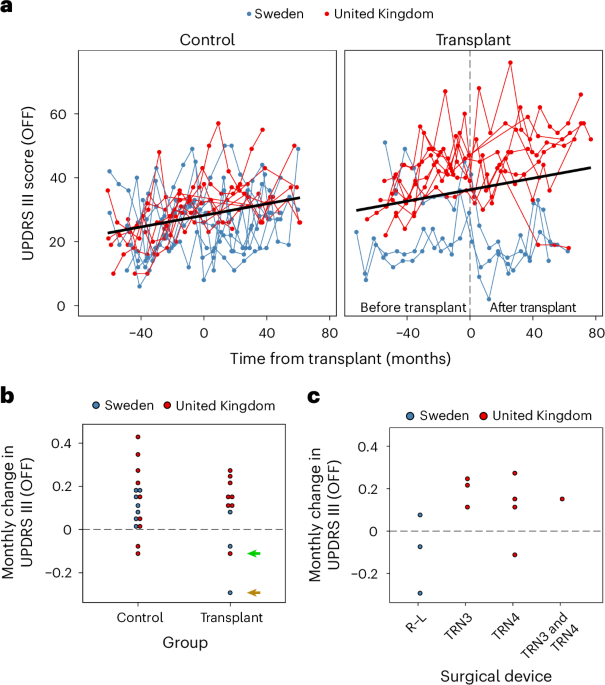
- A new trial called TransEuro was conducted between 2015-2018 in the UK and Sweden involving 11 patients receiving hfVM transplants, with a control group of 16 non-transplanted individuals. Patients were followed for 3 years.
- Main findings:
– No consistent clinical benefit observed across the transplanted cohort when compared to baseline scores or controls.
– Positive correlation noted between surviving dopamine cells and improved PET imaging outcomes in select individuals.
– Differences in surgical site outcomes observed; Lund patients showed better responses possibly due to devices or patient-specific factors. Further statistical analysis wasn’t feasible due to small sample sizes.
– Secondary outcomes indicated improvements for some transplanted individuals regarding reduced medication dosages and less reported OFF time (severity of symptoms without medications).
– PET imaging showed stabilization of dopamine signal levels post-transplantation in specific brain regions but no overarching advancement across all subjects.
Read more: Source Link
Indian Opinion Analysis
The TransEuro study underscores both the promise and challenges of neural transplantation as a therapeutic strategy for Parkinson’s disease. While individual successes highlight potential merits, broader unpredictability hinders its feasibility as a standard treatment approach. For India-a nation grappling with increasing prevalence rates of neurodegenerative diseases-this research demonstrates the importance of investing not only in advanced neuroscience but also parallel healthcare tools focused on risk stratification and adverse effect minimization.
The trial results suggest that refined surgical protocols combined with multidisciplinary optimization might be necessary steps before routine application can be considered even globally. In India’s context, scaling such therapies would demand substantial advancements in medical infrastructure alongside regulatory frameworks tailored for ethical concerns related to fetal tissue use.
Ultimately, while fostering continued participation in global studies could help strengthen India’s expertise base, policymakers must weigh such innovation against population-wide access measures prioritizing early diagnostics and cost-effective interventions within neurocare systems.Quick Summary:
- Fetal cell transplantation for Parkinson’s disease (PD) was studied on 11 transplant patients and 16 control patients in the TransEuro trial.
- Dopaminergic PET imaging suggested marginal improvements in dopamine levels post-surgery but showed notable variability among patients. Only one transplant patient achieved near-normal dopamine signaling.
- Neurological adverse events were considerably higher in the transplant group; thes included dyskinesias, with mild side effects observed in 27% of grafted individuals. Two cases experienced intracerebral hemorrhages during surgery but recovered without lasting complications.
- Serious adverse events related to immunosuppression and surgery occurred in five transplant recipients, including wound dehiscence, colitis, and Kaposi’s sarcoma; no deaths occurred during the study period.
- The transplanted tissue ofen lacked consistency due to variable gestational age of donor material,insufficient standardization procedures,and poor surgical targeting methods leading to misplacement in one case.
Indian Opinion Analysis:
The findings represent a critical challenge for relying on fetal cell transplantation as a viable therapeutic option for Parkinson’s disease treatment within clinical settings. Despite decades of research initiatives attempting cellular therapies, this trial underscores limited efficacy stemming from inconsistent tissue inputs or surgical techniques resulting in variable patient outcomes-even where neurological improvements were observed incrementally over months post-transplantation.
India has vast talent within neuroscience R&D which alongside embracing practical lessons derived global experiments may collaboratively invest exploration yielding less-dependence harvested fetal implants pivot focus cultivation automated precision-designed therapeutics modulating Parkinson Disease substitutable replicable standards evolving regenerative ecosystem models generations usable wider-reach conditions maintenance tailored frameworks adjusting protocols crossings bridging comparative gaps! For details visit full complete source:Click Deployments rpt full exploratoryQuick summary
The TransEuro trial underscores both the promise and challenges of cell-based therapies for parkinson’s Disease. While hfVM transplantation demonstrated some potential improvements in treated patients’ symptoms, significant limitations-such as uncertain consistency of fetal tissue sources-highlight practical barriers impacting scalability and broader applicability. Globally advancing stem-cell-derived dopaminergic therapies may address these issues by enabling batch-controlled production processes with standardized quality metrics.India could benefit thru active participation in international collaborations that allow access to clinical technologies derived from newer regenerative medicine models. Additionally, lessons learned highlight the importance of ethical frameworks regulating cellular therapies-a consideration especially relevant given India’s interest in accelerating medical innovation while balancing cultural sensitivities. Read MoreQuick summary Indian opinion Analysis Read more: Nature Article Link: www.nature.com/articles/cas-redirect Stem-cell-based innovations signal promising developments in treating degenerative neurological diseases like Parkinson’s. India, being home to emerging biotech hubs, could benefit from integrating advancements revealed in global research into its own medical landscape. Having centers equipped with cutting-edge technology such as PET imaging could help India foster early diagnostics and monitor progression in neurodegenerative disorders efficiently. moreover, considering the ethical implications associated with the use of embryonic resources outlined globally, Indian stakeholders might need discussions across scientific bodies to align local laws before importing or developing similar solutions domestically.This case further emphasizes India’s role as an active participant or contributor within neuroscience collaborations due to its access to a diverse patient pool perhaps benefiting translational medicine efforts. Link: www.nature.com/articles/cas-redirectquick Summary: Indian Opinion Analysis: Read moreQuick Summary Indian Opinion Analysis
Indian Opinion Analysis
This research demonstrates advancements in stem-cell therapy for diseases like Parkinson’s but highlights challenges in ensuring consistent efficacy due to patient heterogeneity and small sample sizes. For india-where accessibility is a key concern-such specialized medical interventions offer hope but raise questions about scalability within a diverse population facing resource constraints in healthcare delivery systems. While promising breakthroughs like these expand global medical knowledge, their significance for India rests on affordability frameworks that prioritize equitable access without compromising scientific integrity or ethical safety standards.Quick Summary
Indian Opinion Analysis
This initiative reflects the growing importance of global collaboration in tackling complex diseases like Parkinson’s. For India-a country grappling with an increasing aging population susceptible to neurodegenerative diseases-such advancements highlight potential pathways for local research integration. India’s robust biotechnology capabilities could enable partnerships in clinical trials or application of findings towards affordable treatment solutions domestically. However, success would demand equal emphasis on ethical considerations surrounding embryonic stem cells as well as enhancement of healthcare infrastructure to ensure wide accessibility if therapies transition into clinical applicability.
This milestone in medical biotechnology marks notable progress toward addressing neurodegenerative diseases like Parkinson’s through innovative approaches such as cell transplantation. India’s growing biomedical research infrastructure could benefit from integrating similar advanced studies to address public health challenges.However, such interventions raise ethical and logistical considerations that need careful deliberation within India’s socio-cultural framework. Supporting scientifically validated trials while ensuring accessibility and inclusivity could enhance the long-term impact of such advancements on healthcare systems globally.